Covid-19: How Likely Is a Second Wave?

Executive Summary
Evidence presented in this paper indicates that the severe acute respiratory syndrome coronavirus 2 pandemic as an event in the UK is essentially complete, with ongoing and anticipated challenges well within the capacity of a normalised NHS to cope. The virus infection has passed through the bulk of the population as a result of wholly natural processes and evidence indicates that in the UK and other heavily infected European countries the spread of the virus has been all but halted by a substantial reduction in the susceptible population. This has occurred because the level of infection required to introduce enough immunity into the population to reduce the reproduction number (R) permanently below 1 occurred at markedly lower infection rates and loss of life than had been initially anticipated. The evidence presented in this paper indicates that there should be no expectation of a large scale ‘second wave’ with smaller localised outbreaks when the virus contacts pockets of previously uninfected populations.
Current mass testing using the PCR test is inappropriate in its current form. If it is to continue, then results and reporting should be refined to meet the gold standard of testing methodology to give clinicians improved information so that they are able to make appropriate clinical decisions. Positive tests should be confirmed by testing a second sample and all positive tests should be reported along with the Cycle Threshold (Ct) obtained during the test to aid assessment of a patient’s viral load.
It is recommended that a greater focus be placed on evidence-based medicine rather than highly sensitive theoretical modelling based on assumptions and unknowns. Current evidence allows for a greatly improved understanding of positive infectious patients and using the evidence to improve measurements and understanding can lead to sensitive measurements of active cases to give a more accurate warning of escalating cases and potential issues and outbreaks.
Background
Based upon guidance from NHS England, our primary and secondary care service across the country are currently following protocols to limit access to care due to the dangers of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 or COVID-19) pandemic. Whilst work has begun to restore NHS services (the “restoration”), there remains a strong focus on preparing for a second wave as implied by the Imperial College epidemiological model designed by Professor Neil Ferguson and his team. While this model may have had some limited value when we were faced with a novel virus outbreak, the evidence that has emerged over recent months along with detailed analysis of previous outbreaks implies that the model that is still being followed is unreliable and not consistent with both previously measured systems and current evidence. This paper outlines the evidence and data we have gathered to support a change in focus to further expedite the return of both primary and secondary care to full capacity.
The COVID-19 pandemic has undoubtedly allowed for some very positive and rapid changes within NHS pathways, protocols and services which should be maintained. However, the current reduction in delivered primary care activity, referrals and elective care gives concern as to the degree of ‘collateral damage’ being caused in patients not receiving the diagnostic and ensuing care they should be receiving at the earliest possible stage of intervention. While there has been a very specific focus on the cancer and cardiology services, similar negative impacts can be seen across most services with, for example, neurological, dermatological and renal patients all presenting with more severe disease due to delays in receiving both diagnosis and treatment.
Mortality and Critical Care
National weekly mortality data is useful for looking at the effect of the COVID-19 pandemic. The past four years data were used for comparison purposes and to calculate upper and lower control limits (based on two standard deviations).
This shows that in the pandemic peak (April 17th to 30th) more than twice the number of seasonal average deaths occurred, with the number of deaths above the upper control limit from March 27th through to June 12th, totalling 44,895 excess deaths. Since June 26th the number of weekly deaths has now fallen so it is not only below the weekly average but has regularly dropped below the lower control limit, showing that we are now at the lowest number of weekly deaths recorded in many years.

Over the last three months since lockdown measures started easing on the May 10th there has been no increase in weekly deaths. On the contrary, these have continued to fall.
Another useful measure of disease impact is the Adult Critical Care Bed Occupancy which showed a peak in bed demand between April 7th and 23rd with the number of patients occupying critical care beds significantly higher than our national baseline capacity. However, by the end of May the occupancy had dropped back to pre-COVID-19 levels, well below the national baseline capacity and has shown no statistical change since.

Restrictions have been progressively eased across the country for over three months. A continuation of the virus would be expected to manifest itself as an increase in both Critical Care bed occupancy and national All-Causes Mortality statistics. This has not been the case in either critical indicator.
A Complete Event of the Pandemic
There are very good reasons to believe that the population of the UK and of many heavily infected countries have arrived at a position where the prevalence of the virus is low and probably falling further because the reproduction number (R) has been below 1 for several months. We understand the term ‘herd immunity’ can raise hackles in some quarters of the media. However, it might be more acceptably expressed by stating that the proportion remaining of the population who are susceptible to the virus has fallen sufficiently far that a sustained and growing outbreak of disease is no longer supported. This end state is not at all new or, in our view, controversial. It is how mammals – specifically jawed vertebrates – learned to live with the thousands of viruses that infect every living organism on the planet, not just us, but even plants, fungi and bacteria.
We are of the view that a continued focus primarily on the virus flows from responding to what we are concerned is a seriously flawed transmission model. We are told that only seven per cent of the population have antibodies to the virus and it is implied that this represents the proportion of the population who have so far been infected. The model assumes that we started with 100% susceptibility, because the virus is new, therefore the virus hasn’t gone away and must sooner or later return. This is the basis of all the second wave fears we hear about.
However, we do not believe the model is correct and our assertions and inferences are based upon recently published science, some in highly eminent journals and some by researchers in pre-review online servers which have this year become crucial in keeping pace with emerging science.
While published data on deaths ‘with’ COVID-19 is dependent on testing regimes and therefore liable to inaccuracy due to missing information – for example undetected asymptomatic patients – the data does allow a sound approximation of the flow of the outbreak. Inspecting the daily COVID-19 deaths vs. time curve for the UK we see a Gompertz-type curve (Rypdal and Rypdal, 2020) which are typical of natural, biological phenomena, well documented in biomedical scientific papers over the last 40 years. Note the lack of discontinuities in the curve, suggesting no effective interventions have interrupted its development.

Epidemic Outbreaks
The Gompertz-type plot seen above, which is formed by a single surge in activity, often followed by smaller minor upturns as the disease reaches new populations is typical of previous virus outbreaks that have been well documented, none of which have demonstrated a significant second wave even though control methods were used to prevent the spread of disease in each case.
For example, below we see in the MERS CoV outbreak of 2015 what appears to be a significant double wave. However, it is actually multiple single waves affecting geographically distinct populations at different times as the disease spreads. In this case the first major peak was seen in Saudi Arabia with a second peak some months later in the Republic of Korea. Analysed individually, each area followed a typical single event Gompertz curve.
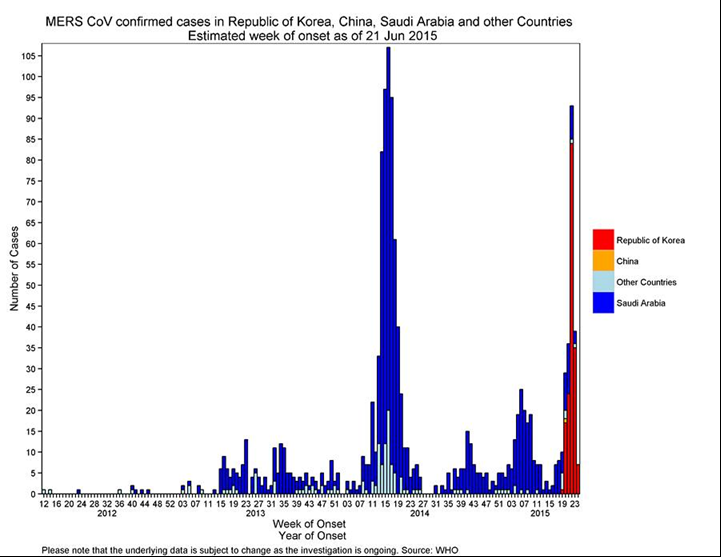

Similarly, when we look at the SARS outbreak of 2003 the initial identification of an apparent double wave when looking at world wide data is actually multiple single events or waves in disparate locations each following the typical Gompertz-type curve.


Population Susceptibility
It is now established that at least 30% of our population already had immunological recognition of this new virus, before it even arrived (Le Bert et al, 2020; Braun et al, 2020; Grifoni et al, 2020). COVID-19 is new, but coronaviruses are not. There are at least four well characterised family members (229E, NL63, OC43 and HKU1) which are endemic and cause some of the common colds we experience, especially in winter.
They all have striking sequence similarity to the new coronavirus. A major component our immune systems is the group of white blood cells called T-cells whose job it is to memorise a short piece of whatever virus we were infected with so the right cell types can multiply rapidly and protect us if we get a related infection. Responses to COVID-19 have been shown in dozens of blood samples taken from donors before the new virus arrived. The most recent paper by Mateus et al (2020) was published in the journal Science in August and supports the previous findings of Le Bert et al (2020).
Importantly, only Mateus performed detailed epitope mapping and found that epitopes present in each of the known endemic coronaviruses share sequence homology or close similarity to those in the new virus. Prior to this, three other groups including immunologists in Germany, Sweden and the USA each independently published similar findings (refs as above and discussed in Sewell, 2020). These papers showed this pre-immunity is geographically widespread and prevalent within each population studied, but it was only the Mateus paper that gave us the understanding as to why and how. It had previously been suggested that pre-pandemic immune responses in circulating T-cells might have occurred following exposure to one or more of the endemic coronaviruses. Mateus, by using parts of these endemic coronaviruses which also exist within COVID-19 confirmed this.
We understand that objections might be raised about the clinical correlates of this T-cell recognition. While that is a fair challenge, it would be unreasonable to dismiss it and assume is has no relevance. This is because this is how T-cell memory works (for example, Ling et al, 2020 show that convalescent COVID-19 patients analogously display exactly these T-cell responses) and more importantly because we have solid evidence in the case of SARS that those expressing T-cell recognition of that coronavirus were resistant to it. In a study of 23 people who survived SARS in 2003, every single one had memory T-cells that recognised the SARS virus 17 years later. (Le Bert et al, 2020). The T-cell response was consistent with measurements taken after vaccination with approved vaccines for other viruses. As important, these T-cell responses also develop even in recovering patients infected with the new virus but who were asymptomatic (Sekine et al, 2020).
In conclusion, we believe it is reasonable to take from this body of work that those displaying vigorous T-cell responses to this family of coronaviruses are resistant to or immune from infection. They are distinct from the others in the population who do not have these T-cell responses and are therefore susceptible to a new virus.
Immunity Threshold
Transmission models, such as the one used by the Imperial team, are highly sensitive to the input parameters they are based on and we argue that a modification of the current model should be applied with, at most, 70% initial population susceptibility. This is a conservative value since current literature finds that between 20% and 50% of the population display this pre-pandemic T-cell responsiveness, meaning we could adopt an initially susceptible population value from 80% to 50%. The lower the real initial susceptibility, the more secure we are in our contention that a herd immunity threshold (HIT) has been reached.
However, our concerns with the Imperial model are not limited solely to T-cell memory mediated reduction in initial susceptibility. This is because there are factors other than T-cell mechanisms which alter a person’s susceptibility to the virus. We now know that children, especially young children, appear harder to infect and/or they are less affected by the virus. To do us harm, viruses need to get inside our cells. To do that, they exploit as ‘grappling hooks’ receptors on the outside of those cells – in the case of the new virus, and at high speed, scientists determined it is an enzyme called ACE2. It turns out that the levels of ACE2 are highest in adults and much lower in children, becoming progressively lower the younger they are (Lingappan et al, 2020). That is a fortunate finding indeed, and goes some way in explaining why children have been relatively spared. In addition, other groups have shown that infectivity is significantly reduced in individuals with the O-blood group (Wu et al, 2020; Ellinghaus et al, 2020). There are approximately eight million children aged 0-10 in the UK and 12.7 million aged 0-15. These cohorts represent approximately 11.9% and 19% of the UK population, respectively
Taking this into account it is, in total, at least 35%, and likely to be significantly more of the population who are resistant or immune to the virus, meaning that they will neither get ill nor participate significantly in viral transmission (Lee, 2020). This is crucial to understanding where we are with respect to the epidemic in the UK and the potential for a second wave of infections.
The proportion of the population that need to be resistant to an infection, in order to stop it spreading, depends on the proportion who were originally susceptible and the initial reproduction number, or R0. If 100% truly were susceptible, then epidemiology suggests that 65% would have to be infected for the herd immunity threshold to be reached, given the initial estimates of R0. That would have resulted in very many more deaths than have been measured. But if, as we are now reasonably sure, a much lower initial percentage was susceptible, it takes far fewer people to catch the virus before there are too few susceptible people remaining within the population for the virus to be able to find the next person to infect.
Recent seroprevalence studies, which measure the proportion of the population displaying antibodies to the novel virus, are widely assumed to show the proportion of the population which has been infected. However, the observation that, for example, only 17% of Londoners have antibodies is not the same as saying only 17% have been infected (though the media often wrongly assumes it does). It is important to appreciate that much of the early serological studies were conducted on hospitalised patients who, by definition, are the most ill cohort. In such patients the majority do seroconvert (eg Theel et al, 2020). In mildly symptomatic and asymptomatic patients, a lower proportion seroconvert (Long et al, 2020). This is because the antibody system is but one of several tools our immunology has to defend us. There have been a number of papers illustrating this important principle. Long et al (2020) find that almost half of previously infected individuals are no longer seropositive a few months later. Gallais (2020) shows that none of the familial contacts of those testing positive to SARS-CoV-2 went onto to develop antibodies.
A reasonable hypothesis is that the lower intensity of immunological challenges tends to rely less on the generation of antibodies and more on innate and cellular responses. This means that a factor of two-fold and possibly higher would need to be applied to population serology data in order to better approximate the infected population. If 7% is the mean for UK, then perhaps 14-21% of the population has actually been infected (which would imply, very approximately, 9-14 million people infected). The authors recognise that the exact number in this example is speculative, but conversations with immunologists indicate that this principle is widely accepted as reasonable for community infection where viral load varies widely and contrasts markedly with seroconversion after vaccination, where the goal is close to 100%.
Interestingly, this question of what percentage of the population have been infected can be approached using a different methodology. Numerous estimates have been made of the infection fatality ratio (IFR) for this new virus. Naturally, it varies depending on the population under study as well as the methodology used and, accordingly, researchers have arrived at a wide range of estimates for IFR. The Centre for Evidence-Based Medicine has done much work in this area and their current estimate is 0.1-0.4% (Oke and Heneghan, 2020). Let us take a midpoint value, especially as for months the US CDC displayed a value for IFR of 0.26% on their website. This implies that for every death from COVID-19, there were a preceding 100/0.26 or ~400 infections. The UK has suffered approximately 42,000 such deaths which, to a first approximation using IFR, implies 16.8million infections, or 25% of the population having been infected.
Consequently, two different and independent analytical approaches provide estimates that are at least in the same range for total population having been infected (overlapping at approximately 20%), and this is crucial in the argument put forward here. Other, theoretical epidemiological studies show that, with the extent of prior immunity that we can now reasonably assume to be the case, only 15-25% of the population being infected is sufficient to bring the spread of the virus to a halt (Lourenco, 2020; Gomez et al, 2020). Importantly, we emphasise there are additional schools of epidemiological work which show that variation in likelihood of becoming infected itself can greatly reduce the so-called herd immunity threshold and that this can be reached at even lower proportions of the population having been infected (e.g. Aguas, 2020).
We saw early on in the pandemic that the number of daily deaths rapidly soar and at that time did we not know where and when it would stop rising. It has been evidenced previously that the most easily infected people got infected earliest (see Gomez et al, 2020). Humans vary hugely, not only in our responses to viruses, but also in the ease or difficulty the virus experiences as it tries to invade us. The most susceptible were those already elderly and/or ill, some very ill, and so we saw very high death rates initially. Once that super-susceptible group were removed from the pool of susceptible individuals by the virus, it began a slower march through everyone else, slowing all the time, as the remaining population’s susceptibility fell continually towards the herd immunity threshold. That is where our evidence indicates we are now and why the virus is disappearing from the environment.
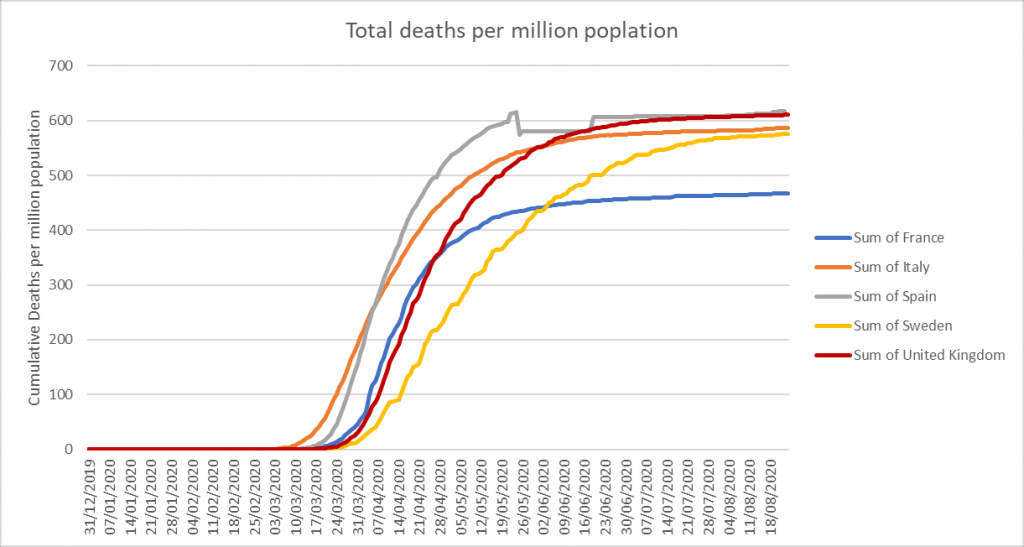
It is important to see this document in light of information available elsewhere in the world. It has widely been observed that in all heavily infected countries in Europe and several of the US states likewise, that the shape of the daily deaths vs. time curves is similar to ours in the UK. Many of these curves are not just similar, but almost super imposable. Italy, France, Spain, Sweden and the UK, for example (OWID, 2020). The shape of the deaths vs. time curve implies a natural process and not one resulting mainly from human interventions, given the widely varying non-pharmaceutical interventions in those countries. Taking this and applying it more widely, the very strong similarities of UK data with that of nearby countries which employed different responses yields another conclusion – that none of the interventions altered the broad course of the pandemic event. Further, it is reasonable to conclude that the pandemic event has ended in those countries, too. Famously, Sweden has adopted an almost laissez faire approach, with qualified advice given, but no generalised lockdowns. Yet its profile and that of the UK’s is very similar. The officials in Sweden appear to be of the view that their population has closely approached or in some places reached what they term herd immunity, with R persistently lower than 1.
The PCR Test
The PCR test for the virus is good enough to confirm infection in someone with symptoms. “Is it flu or is it COVID-19?” is a question easily answered. What it is very poor at, however, is what is being asked of it now, namely estimating the percentage of people who are currently infectious in the community. We do not know exactly what the false positive rate is, but it is widely believed to be greater than the actual, remaining prevalence of the virus (Heneghan, 2020), which is around 1:2000, or 0.05%. (ONS prevalence survey Aug 14th 2020). The result of continuing to use this test alone on a massive widescale screening program is inevitably to generate a high proportion of false positives. The problem of using any assay to conduct surveillance on a low prevalence virus with a PCR test has been widely discussed (Heneghan, 2020). Under present parameters, even accepting an unlikely 0.1% False Positive rate and a prevalence of 0.1%, more than half of the positives are likely to be false, potentially all of them. It is the opinion of the authors that the false positive rate is higher and the prevalence lower than this. Consequently, it is impossible for the positives to be much other than false. A recent letter to the British Medical Journal (Healy, 2020) exemplifies the extent of harm that actually arose in a setting in which all but one of the positives ended up being false positives. This resulted not only in considerable time and money wasted by surgeries, but also other medical issues being delayed. It is not rational and may even be dangerous to use these results to drive policy. Note that recent so-called ’spikes’ were never accompanied or followed by people getting ill, going to hospital and dying in elevated numbers. Consequently, it is possible that most of the positives from mass testing are either false positives or ‘cold positives’ (fragments of real virus which are not intact and incapable of replication or of causing disease or infecting others) and therefore begs the question of whether mass testing of patients without symptoms is in fact helpful or misleading? It may be of relevance to note that, on August 24th the US CDC changed its guidance on when PCR testing is appropriate. They now recommend not testing people with no symptoms who are not contacts in a contact-tracing activity.
There are practical alternatives to mass testing. For example, calls to the NHS111 service captures all reports of what is termed ‘influenza-like illness’. Change in this parameter is likely to be a much more sensitive measure of the presence of increasing prevalence of SARS-CoV-2 infection than flawed PCR testing without modifications. Obviously, and perhaps it has already happened, there is the potential for emerging influenza to complicate the picture. A modification to the strategy involving PCR testing which would easily resolve any uncertainty is this: every positive test result is followed up as quickly as possible, ideally within 24 hours of the positive result, and every one is retested. If this is done, almost all the false positives will be removed. We predict there would be few genuine positive results remaining. But even here, it is important to recall what it is that the PCR test measures, and it is simply the presence of partial RNA sequences present in the intact virus. This means that even a true positive does not necessarily indicate the presence of viable virus. In limited studies to date, many researchers have shown that some subjects remain PCR-positive long after the ability to culture virus from swabs has disappeared. We term this a ‘cold positive’ (to distinguish it from a ‘hot positive’, someone actually infected with intact virus). The key point about ‘cold positives’ is that they are not ill, not symptomatic, not going to become symptomatic and, furthermore, are unable to infect others. As each PCR test that is carried out returns the Cycle Threshold (Ct) used to obtain a positive result, it is important that this Ct is reported with every positive result. The Ct gives strong evidence of the viral load and aids clinicians in determining if a patient has a “hot” infectious positive result or a “cold” non-infectious positive result. Gniazdowski et al (2020) studied 161 positive PCR test samples with a Ct value below 23 that yielded 91.5% of virus isolates and the study showed a strong correlation between recovery of SARS-CoV-2 infectious virus on cell culture and Ct values. Ct values above 30 returned negative cultures in all except one case.

Expectations of a Second Wave
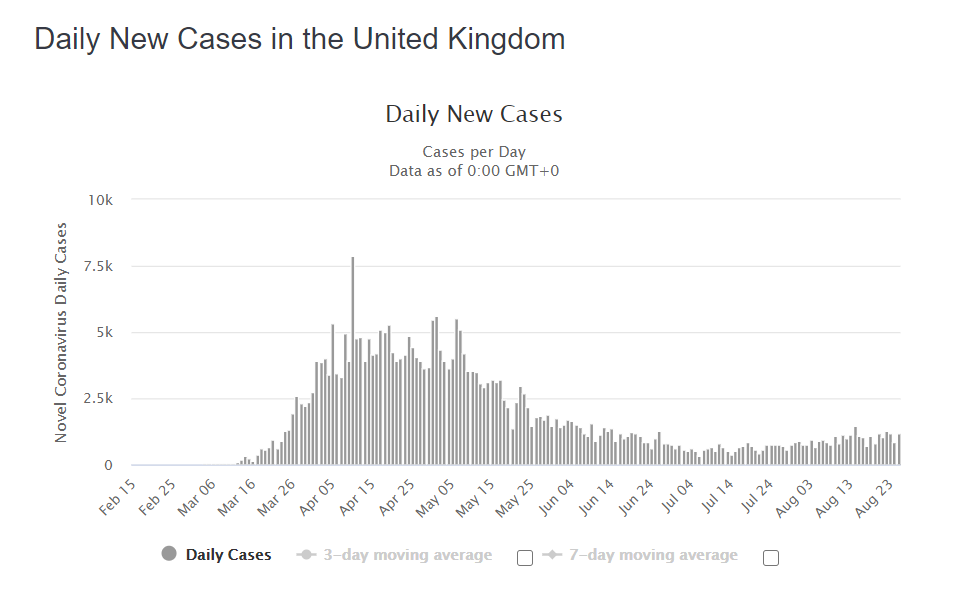
Daily deaths from and with COVID-19 have almost ceased, having fallen over 99% from peak. All the numbers monitored carefully fall like this, too: the numbers being hospitalised, numbers in hospital, number in intensive care – all are falling in synchrony from the April peak. Viral evidence historically tells us that you don’t generally get infected by the exact same virus twice, certainly not within a short period of time. It’d be a poor immune system which lets that happen and we’d probably not have made it as a species into the 21st century if that’s how it worked. So there’s an expectation of some duration of immunity. It needs studying, but our experience and evidence for coronaviruses (Le Bert et al, 2020) suggests that if you have memory T-cells, durability can be very long lasting. This study showed that people still had robust T-cell responses in 2020, 17 years after the first SARS outbreak back in 2003. The concerns people have expressed about falling antibody levels underscores a lack of knowledge about acquired immunity. It is not efficient nor required for immunity to maintain high levels of antibodies to everything to which you are immune. Instead, cellular memory enables very rapid re-generation of antibodies upon re-encounter with the antigen, if that is required to defend the host. Alternatively, innate and cellular memory responses can be sufficient.
The NHS currently remains ‘COVID-19 ready’ in preparation for an expected second wave, a highly unlikely scenario based upon an initial model with highly sensitive input variables that we already know to be inaccurate. The evidence we’ve presented leads us to believe there is unlikely to be a second wave and that while there have been apparent multi-‘wave’ respiratory viruses in the past, notably 1918-20, in many cases it became clear that this was either different populations being infected at different times or in some cases multiple different organisms involved. There is no biological principle that leads us to expect a second wave based on the accumulation of data over the past six months. Instead, it is likely there will be local, small and self-limiting mini-outbreaks as areas previously unexposed come into contact with the virus.
Spain and France
So what is happening in terms of second wave concerns in France and Spain? As the rate of hospitalisations, ICU utilisation and the daily death rate from COVID-19 all decayed steadily, it appears that several but not all countries have greatly expanded their testing capacity in the broader population of people who are not showing any symptoms of infection. We contend that the many claims in the media for outbreaks, spikes and second waves are all artefacts of amplified rates of testing. It should be noted that illness, hospitalisations and deaths have not reversed in any clear and sustained manner. Specifically, careful examination of the weekly all-causes mortality data in France is completely clear. Six weeks into an apparent surge of cases, the number of deaths remain completely flat and normal, in all age bands (as of mid-August when this document was written).






*
Note to readers: please click the share buttons above or below. Forward this article to your email lists. Crosspost on your blog site, internet forums. etc.
Paul Kirkham, Professor of cell Biology and Head of Respiratory Disease Research Group at Wolverhampton University
Dr Mike Yeadon, former CSO and VP, Allergy and Respiratory Research Head with Pfizer Global R&D and co-Founder of Ziarco Pharma Ltd
Barry Thomas, Epidemiologist
Sources
Aguas, et al (2020). Herd immunity thresholds for SARS-CoV-2 estimated from unfolding epidemics. medRxIV https://doi.org/10.1101/2020.07.23.20160762.
Braun, et al. (2020). Presence of SARS-CoV-2 reactive T cells in COVID-19 patients and healthy donors. medRxIV https://doi.org/10.1101/2020.04.17.20061440
Ellinghaus et al. (2020) Genomewide Association Study of Severe Covid-19 with Respiratory Failure. New Eng. J Med. DOI: 10.1056/NEJMoa2020283
Gallais, (2020). Intrafamilial exposure to SARS-CoV-2 induces cellular responses without seroconversion. medRxIV https://doi.org/10.1101/2020.06.21.20132449.
Gomez et al. (2020). Individual variation in susceptibility or exposure to SARS-CoV-2 lowers the herd immunity threshold. MedRxIV https://doi.org/10.1101/2020.04.27.20081893
Grifoni et al. (2020. Targets of T Cell Responses to SARS-CoV-2Coronavirus in Humans with COVID-19Disease and Unexposed Individuals. Cell 181, 1489–1501. https://doi.org/10.1016/j.cell.2020.05.015ll
Healy, B (2020). Covid-19 testing, low prevalence and the impact of false positives. Brit Med J. 2020;369:m1808.
Long, et al. (2020). Clinical and immunological assessments of asymptomatic SARS-CoV-2 infections. Nature Med 26, 1200-04.
Heneghan (2020). How many Covid diagnoses are false positives? The Spectator, July 20 2020.
Le Bert et al (2020) SARS-Cov-2 specific T cell immunity in cases of Covid19 and SARS and uninfected controls. Nature. Doi 10.1038/s41586-020-2550-z
Lee & Raszka (2020). COVID-19 transmission and children: the child is not to blame. Pediatrics, e2020004879 DOI: 10.1542/peds.2020-004879.
Ling, et al (2020). Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity 52(6), 971-77.
Lingappan et al (2020). Understanding the age divide in COVID-19: why are children overwhelmingly spared? Am. J. Physiol (Lung Cell Molec. Physiol) https://doi.org/10.1152/ajplung.00183.2020
Lourenco et al (2020). The impact of host resistance on cumulative mortality and the threshold of herd immunity for SARS-CoV-2. MedRxIV https://doi.org/10.1101/2020.07.15.20154294
Mateus et al (2020) Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. DOI: 10.1126/science.abd3871
Oke& Heneghan (2020). Global Covid-19 Case Fatality Rates. https://www.cebm.net/covid-19/global-covid-19-case-fatality-rates/
June 9 2020 update.
ONS coronavirus survey (Aug 14 2020). https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveypilot/latest
OWID (our world in data: display UK, Sweden & France, confirmed daily deaths, log plot per million) https://ourworldindata.org/coronavirus-data-explorer?yScale=log&zoomToSelection=true&time=2020-03-02..latest&deathsMetric=true&interval=smoothed&aligned=true&perCapita=true&smoothing=7&country=TWN~GBR~SWE~FRA&pickerMetric=location&pickerSort=asc
Rypdal & Rypdal (2020) A parsimonious description and cross-country analysis of COVID-19 epidemic curves. https://arxiv.org/pdf/2008.02475.pdf
Sekine et al. (2020). bioRxiv preprint doi: https://doi.org/10.1101/2020.06.29.174888.
Sewell, H. (2020). BMJ 2020;370:m3018.
Theel et al (2020). The Role of Antibody Testing for SARS-CoV-2: Is There One? J. Clin Microbiol, 28(8), 1-7.
Wu et al (2020) Association between ABO blood groups and COVID-19 infection, severity and demise: A systematic review and meta-analysis. Infection , Genetics and Evolution. Doi 10.1016/j.meegid.2020.104485
Wood (2020). Did COVID-19 infections decline before UK lockdown? https://arxiv.org/pdf/2005.02090.pdf
Gniazdowski V, Morris P, Wohl S et al. Repeat COVID-19 molecular testing: correlation with recovery of infectious virus, molecular assay cycle thresholds, and analytical sensitivity. medRxiv 2020.08.05.20168963; doi: https://doi.org/10.1101/2020.08.05.20168963
Other Data sources and Reference
https://github.com/owid/covid-19-data/find/master
https://en.wikipedia.org/wiki/2012_Middle_East_respiratory_syndrome_coronavirus_outbreak
https://en.wikipedia.org/wiki/2002%E2%80%932004_SARS_outbreak
https://science.sciencemag.org/content/303/5664/1666
https://www.worldometers.info/coronavirus/country/spain/
https://www.worldometers.info/coronavirus/country/france/
https://www.gov.uk/guidance/coronavirus-covid-19-information-for-the-public#number-of-tests
Featured image is from LS
