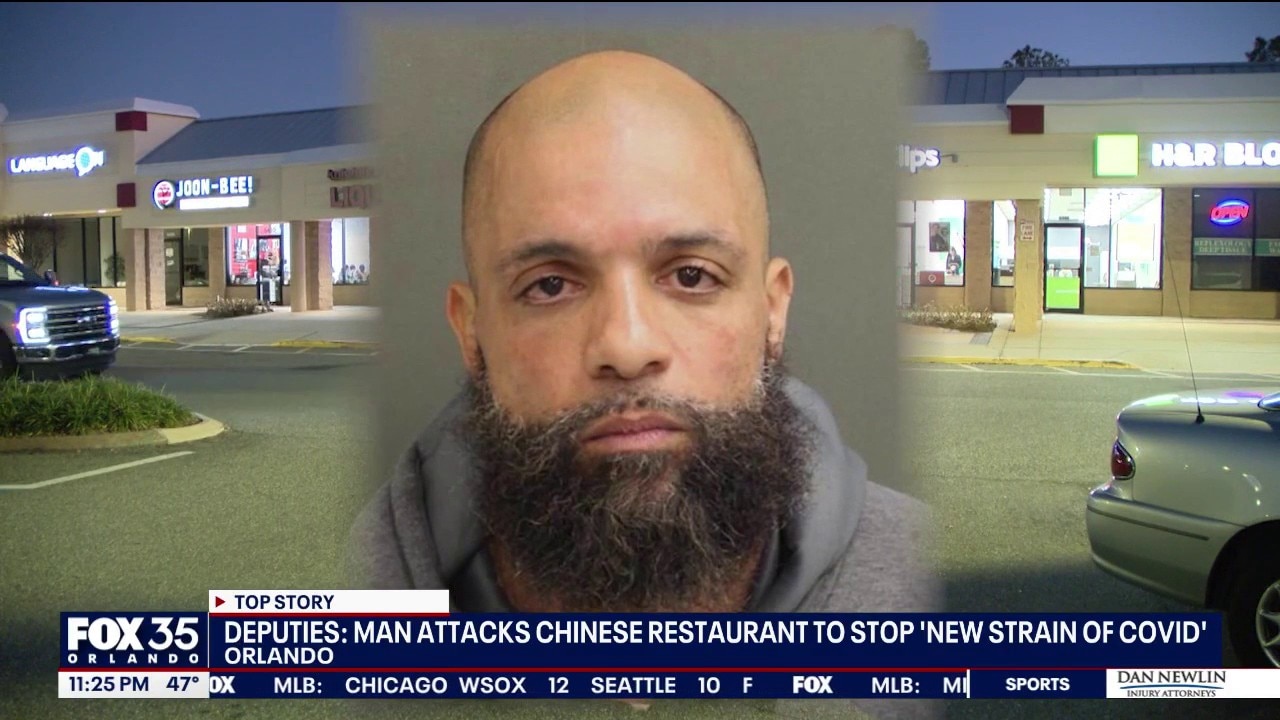
‘Average’ effectiveness & unexplained side effects: 7 QUESTIONS that AstraZeneca needs to answer about its Covid-19 vaccine

The drug manufacturer AstraZeneca said on Monday it would seek emergency authorization for its coronavirus vaccine with various regulators. However, it might first have to answer a number of serious questions about its jab.
The British-Swedish company, which has developed its candidate vaccine together with the University of Oxford, “will begin the submission of the clinical data to regulators around the world that have a framework in place for emergency use or conditional approval,” its spokesman said on Monday, adding that the list of the regulators that will receive the application particularly includes the US Food and Drug Administration (FDA).
The statement came as the drug manufacturer published interim results of the vaccine’s clinical trials, which it said“showed the vaccine was highly effective in preventing COVID-19” and declaring its overall effectiveness to amount to 70 percent – much lower than 90-percent efficacy that other leading vaccine candidates showed.
Yet, some information published by the company appeared to be quite puzzling, while some data seemed to be lacking.
AstraZeneca might eventually find itself in need of answering key questions about its would-be jab as it rushed to get regulators’ approval for it.
1.Half dose or full dose?
The company’s assessment of its vaccine effectiveness in fact comes as an estimated average for two slightly different tests. AstraZeneca admitted in its Monday release that a group of volunteers initially got a half-dose jab, followed by afull one a month later. Another, larger, group got two full doses.
The first regimen showed 90-percent efficacy, although such results were based on analysis of slightly more than 2,700 volunteers. The second one, which involved almost 8,900 people, showed only a 62-percent efficacy.
Such a discrepancy shows that AstraZeneca has two approaches to its vaccine use, demonstrating quite different results, and that it may probably need to file for approval of each one separately.
Which one would it choose, if any? How long will the new clinical trials take, as results from these will be needed for any dose changes? Or will it stick to its “average” assessment approach?