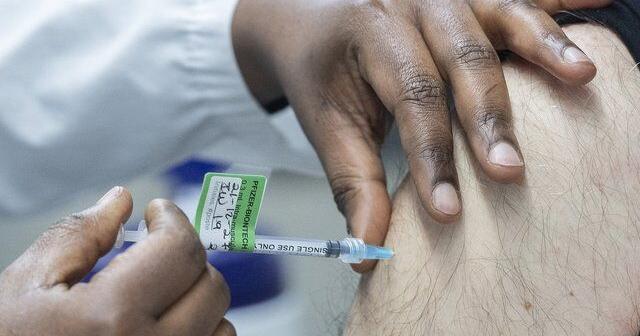
India launches vaccine drive as scepticism mounts
Narendra Modi has kicked off one of the world’s most ambitious inoculation drives in the midst of growing vaccine scepticism over the contentious approval of an indigenously developed jab.
The Indian prime minister launched the campaign with an emotional live address on Saturday, saying “the nation has been desperately waiting for this moment” and warned against “false propaganda” about vaccine safety.
India, a country of 1.4bn people, has the world’s second-highest number of coronavirus infections at 10.5m. Lockdowns have had limited effect in controlling the spread of the virus and contact tracing has faltered, making a successful inoculation programme essential. The first phase of the vaccination rollout targets 30m healthcare and frontline workers, with the goal of inoculating 300m people by July.
The outcome of the drive will determine if India can inject confidence into an economy that has struggled to recover following one of the world’s most severe and disruptive lockdowns. After a gruelling recession, growth remains tepid and millions of Indians have been plunged into poverty.
At a Max Healthcare hospital in Saket, a neighbourhood in New Delhi, Chacko, a 42-year-old nurse in the emergency department in mint-green scrubs said he was “was happy to be the first to take the shot. This will change our lives.”
But New Delhi’s approval of Covaxin, a vaccine developed by Hyderabad-based Bharat Biotech, before phase 3 trial data was released has been criticised by healthcare experts and stoked vaccine scepticism. A survey this month by pollster Local Circles found that 69 per cent of respondents were hesitant about getting a jab.
“I think it’s impossible to develop a vaccine in one year,” said Ravi Agarwal, a 45-year-old who runs a small kiosk selling tobacco and sweets in New Delhi. “My son has a blood disease. If they ask me to inoculate him, I would be very worried.”
As was the case with Russian and Chinese vaccines, Covaxin was fast-tracked without completing phase 3 trials and with little data on efficacy. Experts argue that the growing doubt surrounding the vaccine, developed in partnership with India’s government, risks undermining the vaccination drive.
Some states are already pushing back: the health minister of Chhattisgarh, for example, has refused to accept Covaxin until the phase 3 trials are completed.
“What Indian regulators have done is pulled us down to Russia and China’s level,” said Dinesh Thakur, a former pharma executive in India who now works as a public health activist in the US.
“India has never had an anti-vax movement until now,” he said. “The country has gotten over polio and smallpox, people are compliant. But this shady process has given rise to anti-vaxxers.”
Civil society activists allege that Bharat Biotech did not follow proper guidelines at a phase 3 trial study site in Bhopal, a city in the central state of Madhya Pradesh, where a trial volunteer died in December.
Jitendra Narwaria, a 37-year-old sawmill labourer, was given the shot in December. “They said that free medicine was being offered that would cure us of our cough and cold,” said Mr Narwaria, who received Rs750 ($10) for taking the jab.
Like other trial subjects, Mr Narwaria said he suffered side effects. “A few days later I started vomiting and I was feeling very weak, too. I went back to the hospital but no one listened to me and I just came back home,” he said.
“We were not given the complete information. If they had told us it was for corona, I would have said no.”
Bharat Biotech has said that it conducts clinical trials “in compliance with the study protocol” and “the focus at all times is on patient safety”. The company said that the death in December was unrelated to the trial.
Krishna Ella, the Bharat Biotech chairman and managing director, rejected criticism of the vaccine. In an interview with India Today TV this week, Mr Ella said he would not mind giving the vaccine to his six-year-old grandson, even though the jab has not yet been approved for children.
Pushing aside the domestic controversy, Mr Modi has hailed the vaccine as a triumph of Indian science and his self-reliant “Make in India” policy. His government is also leveraging the country’s vast manufacturing capacity to supply the developing world with shots.
On Tuesday, Bharat Biotech announced that it had signed a deal to supply vaccines to Brazil’s Precisa Medicamentos, a drug company. The Serum Institute of India, the world’s largest vaccine manufacturer, has a contract with the UN-backed vaccine alliance Gavi to provide 200m doses of the Oxford/AstraZeneca vaccine.
In New Delhi, healthcare workers welcomed the vaccine with relief. At Max Healthcare hospital, staff were recovering from the city’s third and deadliest wave of coronavirus cases in November after the festival season. Officials warn against complacency even though the capital region’s active caseload has fallen from a peak of more than 40,000 to less than 3,000.
Coronavirus business update
How is coronavirus taking its toll on markets, business, and our everyday lives and workplaces? Stay briefed with our coronavirus newsletter.
“The only end to the pandemic is masks and vaccination,” said Dr Sandeep Budhiraja, group medical director at the hospital.
“Delhi is probably out of the worst of it, but there is no way to rule out a next wave. Look at what is happening in the UK. It can happen anywhere in the world.”