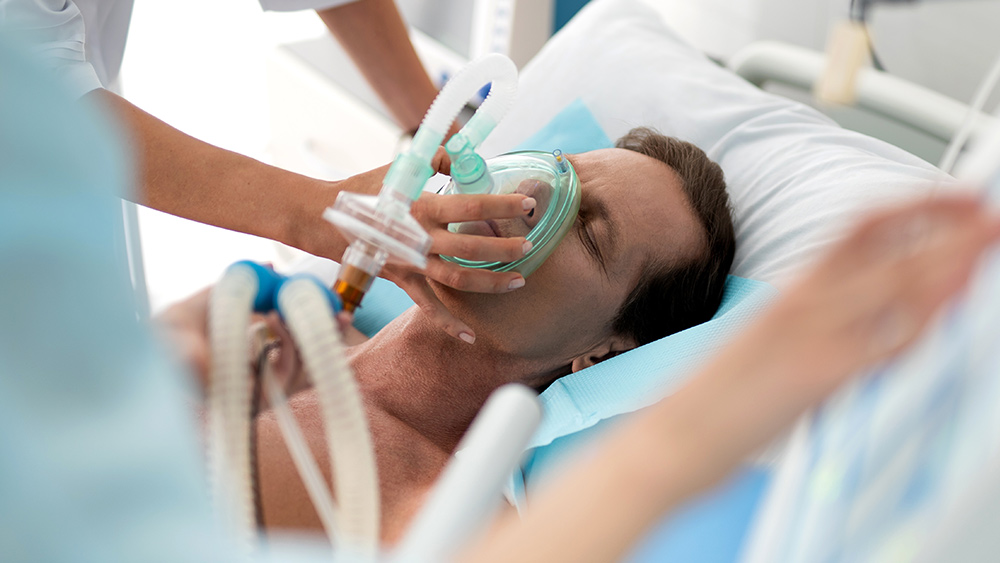
Severe Reactions in Healthy Teens from COVID-19 Shot

17-year-old Everest Romney of Draper, Utah, developed blood clots in his brain within days of receiving a COVID-19 vaccine
Emma Burkey, an 18-year-old from the Las Vegas area, received the Johnson & Johnson vaccine March 20, 2021, and was put into a medically induced coma within two weeks due to seizures and blood clots in her brain
Another devastating report states that a 15-year-old boy from Colorado, with no preexisting conditions or allergies, died from cardiac failure two days after receiving Pfizer’s COVID-19 vaccine
Victims’ families are hoping to receive help for medical bills, but they’re unlikely to get it; if you or a loved one dies or is permanently injured by an experimental COVID-19 vaccine, you cannot sue the drug company that made it
*
Healthy teenagers have been hospitalized,1 and at least one death in a teen has been reported,2 following experimental COVID-19 vaccinations being distributed under an Emergency Use Authorization (EUA) granted to vaccine manufacturers by the U.S. Food and Drug Administration. The adverse events are especially tragic since COVID-19 has a 99.997% survival rate among children and teens,3 making the necessity of vaccination highly questionable.
One of the risks of receiving an experimental medical procedure like a COVID-19 vaccine is that each person who participates is part of the experiment. Unexpected adverse reactions can and do occur, even with vaccines that have been in use for decades.
Often, the reactions may be mild, including symptoms such as headaches, muscle pain, chills and fever, but in other cases, the reactions may be severe, debilitating and even deadly.
As of April 30, 2021, 3,837 reports of death were submitted to the U.S. Vaccine Adverse Event Reporting System (VAERS).4 Past investigations have shown only between 1%5 and 10%6 of adverse reactions are ever reported to VAERS, which is a passive, voluntary reporting system, so the actual number could be much higher. One study funded by the U.S. government and published in 2011 found that less than 1% of vaccine adverse events are ever reported to VAERS.7
After Shot, Healthy Teen Develops Blood Clots in the Brain
April 21, 2021, 17-year-old Everest Romney of Draper, Utah, received a COVID-19 vaccine. The next day, his neck became swollen and he developed severe headaches, which persisted for days. “He could not move his neck without the assistance of his hands,” his mother, Cherie Romney, told ABC 4 News.8
Everest’s pediatrician initially said the neck symptoms were due to a pulled muscle, but Everest also developed a fever, prompting his mother to push for answers.
The pediatrician prescribed antibiotics and a neck brace, suggesting it may be due to an injury from the basketball Everest plays, but Cherie pushed for a CT scan after migraines continued for more than a week, which revealed two blood clots in his brain and a third on the outside of his brain.
After spending time in the intensive care unit, Everest was discharged but swelling persisted in his eyes and they’re not sure what the future will bring. “The hardest thing was I let him get that shot. And he was healthy and well before,” Cherie said. “But you question it, you can’t help but question it when it all goes wrong … It was pretty awful.”9
18-Year-Old Hospitalized With Blood Clots After COVID Shot
Emma Burkey, an 18-year-old from the Las Vegas area, also developed blood clots in her brain following a COVID-19 vaccine. She received the Johnson & Johnson/Janssen vaccine March 20, 2021, and was put into a medically induced coma within two weeks due to seizures and blood clots in her brain.
She is making a recovery in a rehabilitation center, but Bret Johnson, Burkey’s minister who was asked to act as spokesman, told Fortune, “We don’t know what’s going to happen with Emma, how long it will it take for her to return to a normal life.”10
April 13, 2021, the U.S. Food and Drug Administration (FDA) announced it would pause the use of the Johnson & Johnson COVID-19 vaccine in the U.S. following reports of six cases of rare and severe blood clots called cerebral venous sinus thrombosis (CVST) combined with low blood platelet levels (thrombocytopenia). One death was reported as a result.11
Together, the condition is known as thrombosis-thrombocytopenia syndrome (TTS). At least nine more cases were reported to VAERS between April 13 and April 23, 2021, all in women between the ages of 18 and 59.12
The experimental Johnson & Johnson COVID-19 vaccine uses a human adenovirus vector to deliver double-stranded DNA for the SARS-CoV-2 spike protein into cells, similar to the AstraZeneca/Oxford University experimental COVID-19 vaccine, which uses a chimpanzee adenovirus vector.13
May 10, 2021, an expert panel in Norway recommended that both AstraZeneca’s and Johnson & Johnson’s COVID vaccines be dropped from the country’s vaccination campaign due to the risk of blood clots.14
Denmark has also rejected Johnson & Johnson’s vaccine for the same reason,15 while in the U.S. the FDA and the U.S. Centers for Disease Control and Prevention (CDC) lifted the pause on the shot and recommended use of the vaccine should resume, stating, “At this time, the available data suggest that the chance of TTS occurring is very low, but the FDA and CDC will remain vigilant in continuing to investigate this risk.”16
However, they did add a warning of the risk in their “Fact Sheet for Recipients and Caregivers,” which states:17
“Blood clots involving blood vessels in the brain, abdomen, and legs along with low levels of platelets (blood cells that help your body stop bleeding), have occurred in some people who have received the Janssen [Johnson & Johnson] COVID-19 Vaccine. In people who developed these blood clots and low levels of platelets, symptoms began approximately one to two-weeks following vaccination.
Most people who developed these blood clots and low levels of platelets were females ages 18 through 49 years. The chance of having this occur is remote. You should seek medical attention right away if you have any of the following symptoms after receiving Janssen COVID-19 Vaccine:
- Shortness of breath
- Chest pain
- Leg swelling
- Persistent abdominal pain
- Severe or persistent headaches or blurred vision
- Easy bruising or tiny blood spots under the skin beyond the site of the injection
These may not be all the possible side effects of the Janssen COVID-19 Vaccine. Serious and unexpected effects may occur.”
COVID Vaccine-Related Death of Teen Reported to VAERS
Another devastating report in VAERS states that a 15-year-old boy from Colorado, with no preexisting conditions or allergies, died from cardiac failure two days after receiving Pfizer’s experimental mRNA COVID-19 vaccine.18
In an interview with Yahoo News, Tom Shimabukuro, deputy director of the Immunization Safety Office at the CDC, was quick to brush off the report, stating:19
“Anyone can make a report, and the information is not verified. If classified as serious, the CDC follows up to get medical records. Some of these reports might be true adverse reactions that are caused by the vaccine, and some of these reports are coincidental health events and not related to the vaccine at all … The benefits of vaccination far outweigh any risks from vaccination.”
At least five deaths have been reported to VAERS following COVID-19 vaccination in the 6- to 17-year-old category.20 Thirteen additional reports of life-threatening injury or permanent disability have also been reported in this age group.21 Despite the unknown and potentially deadly risks, COVID-19 vaccines are being tested on children as young as 6 months old.22
Researchers at Yale School of Medicine are leading Moderna’s clinical trial of a COVID vaccine for children 6 months to 12 years old, which is being conducted on 6,750 children at 90 sites in the U.S. and Canada. But as noted by Dr. Inci Yildirim, associate professor of pediatrics (infectious diseases) at Yale School of Medicine:23
“A clinical trial for a children’s COVID-19 vaccine requires the consideration of many additional factors. Children are not little adults. As children grow and develop, their immune system grows and develops. A 16-month-old is not the same as a 16-year-old. They are both children, but their capacity to respond to the vaccines is not the same.”
Victims Looking for Help With Medical Bills, Unlikely to Get It
Burkey, the teen who ended up in an induced coma after vaccination due to blood clots and seizures, has medical bills of $513,000, and that’s just the first round.24 In the U.S., COVID-19 vaccine makers already have something of a “free pass” when it comes to vaccine injury liability and lawsuits under the Public Readiness and Emergency Preparedness (PREP) Act, passed in 2005 and amended in 2020.25
In 1986, the U.S. Congress created a federal no-fault vaccine injury compensation program (VICP) as an administrative alternative to a lawsuit for injuries and deaths caused by vaccines recommended by the CDC for children in the 1986 National Childhood Vaccine Injury Act.26
Over a period of 30 years, that law was weakened with congressional amendments and federal agency rulemaking, as well as a U.S. Supreme Court ruling in 2011 that effectively removed all liability from vaccine manufacturers.
Contested vaccine injury claims filed under the 1986 Act are adjudicated by special masters in the U.S. Court of Federal Claims in Washington, D.C., and there is a trust fund out of which claims are paid, sparing insurance companies representing vaccine makers and vaccine providers from costly payouts for vaccine injuries and deaths.27 Only injury claims for vaccines routinely recommended by the CDC may be heard in this “vaccine court” created in the 1986 Act.
However, the U.S. Court of Federal Claims will not be involved in ruling in contested COVID-19 vaccine injury claims. The previously mentioned PREP Act, which was passed by Congress in 2005 and amended in 2020 with plenty of pharmaceutical industry influence, will separately deal with COVID-19 vaccine injury claims routed through the Countermeasures Injury Compensation Program. As noted by Fortune:28
“The Countermeasures Injury Compensation Program, run by an obscure office within the U.S. Health and Human Services Department, covers medical costs and lost wages not paid by insurance. Some 445 claims had been filed as of April 26 for adverse reactions to either vaccines or treatments, according to the Health Resources and Services Administration [HRSA], which runs the program.”
Of these 445 COVID-19 related claims, about one-quarter are linked to vaccines, and so far no payouts have been received. While HRSA stated that no claims have been compensated because they don’t have all of the required information, the program has a notoriously low rate of compensation.
In the last decade, only 39 of nearly 500 claims filed under the PREP Act have received federal compensation, most often from reactions caused by the H1N1 vaccine.29 The bottom line, sadly, is this, as noted by Barbara Loe Fisher of the National Vaccine Information Center:
“Already wealthy drug companies were given at least $9 billion from the government to develop experimental COVID vaccines in record breaking time,30 shaving five to 10 years off the normal vaccine development, testing and licensing process.31,32
But that wasn’t enough. Congress also handed companies a liability shield from lawsuits whenever the product government paid them to produce fails to work as advertised or a person is hurt by using it.33
If you or a loved one dies or is permanently injured by an experimental or soon-to-be FDA licensed COVID vaccine, you cannot sue the drug company who made it, even if there is evidence the company could have made it less reactive or more effective.”
The National Vaccine Information Center (NVIC) recently posted more than 50 video presentations from the pay-for-view Fifth International Public Conference on Vaccination held online October 16 to 18, 2020, and made them available to everyone for free.
The conference’s theme was “Protecting Health and Autonomy in the 21st Century” and it featured physicians, scientists and other health professionals, human rights activists, faith community leaders, constitutional and civil rights attorneys, authors and parents of vaccine injured children talking about vaccine science, policy, law and ethics and infectious diseases, including coronavirus and COVID-19 vaccines.
In December 2020, a U.K. company published false and misleading information about NVIC and its conference, which prompted NVIC to open up the whole conference for free viewing. The conference has everything you need to educate yourself and protect your personal freedoms and liberties with respect to your health.
Don’t miss out on this incredible opportunity. I was a speaker at this empowering conference and urge you to watch these video presentations before they’re censored and taken away by the technocratic elite.
*
Note to readers: Please click the share buttons above or below. Follow us on Instagram, @crg_globalresearch. Forward this article to your email lists. Crosspost on your blog site, internet forums. etc.