Why the Lyme disease vaccine the US developed went off the market
- Since 1998, confirmed Lyme disease cases in the US have risen roughly 40%.
- GlaxoSmithKline developed a Lyme vaccine in the 1990s. It went off the market by the early 2000s.
- A new anti-Lyme injection (not a vaccine) is coming. But it could take years to be fully approved.
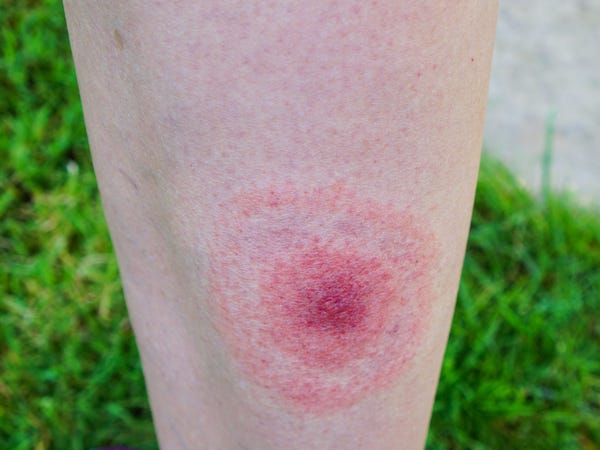
Lyme disease, transmitted by deer ticks and characterized by a hallmark bullseye rash, is an ever-present threat for hikers, hunters, and pet owners. If left untreated, it can cause temporary facial paralysis, shooting pain, arthritis, and speech and memory problems, according to the Mayo Clinic.
Lyme was once considered a regional concern isolated to the Northeast, but climate change has broadened its territory to 44 states. Rising temperatures have expanded ticks’ habitats, studies show, and shorter winters may allow ticks to be active for longer periods during the year.
In 1998, fewer than 17,000 Lyme disease cases were confirmed in the US. Two decades later, that yearly count has increased roughly 40%, with between 20,000 and 30,000 cases reported to the Centers for Disease Control and Prevention. The number of estimated yearly cases, meanwhile, has ballooned to 476,000 people diagnosed and treated for Lyme disease, according to the CDC. (That includes people presumed to have the disease and treated without an official diagnosis.)
But the US had a Lyme vaccine more than 20 years ago: a shot called LYMErix. It seemed to ward off the disease among the small group of Americans who received it — until a perfect storm of dismissive public-health experts, conspiracy theories, a class-action lawsuit, and a lack of consumer demand drove it off the market.
GlaxoSmithKline developed a safe, effective Lyme vaccine decades ago
Pharmaceutical giant GlaxoSmithKline (GSK), known as SmithKline Beecham until a 2000 merger, developed LYMErix in the 1990s. The Food and Drug Administration approved the shot in 1998.

The three-dose vaccine worked by stimulating disease-fighting antibodies in human blood. If a black-legged tick bit a vaccinated person, the antibodies attacked the Borrelia burgdorferi bacteria in the tick’s gut, preventing it from being transmitted to the human host.
The vaccine was about 75% effective at preventing Lyme disease after three doses in clinical trials.
But a CDC committee thought the vaccine was unnecessary
Almost as soon as it was approved, though, LYMErix developed a reputation problem.
Lyme disease was initially detected and most prevalent in New England, and cannot be spread from person to person. So those most at risk tended to be people with access to the outdoors along the coast. Some doctors, then, saw LYMErix as a vaccine developed for and benefitting rich, white people. During a 1998 CDC advisory committee meeting, Dr. Chinh Le of Kaiser Permanente Medical Center described the shot as a “yuppie vaccine.”
Le suggested the shot was being marketed to suburbanites who “will pay a lot of money for their Nikes and their Esprit, and shop at L.L. Beans, will have no consideration for cost-effectiveness when they want a vaccine, because they’re going to travel to Cape Cod.”
US health officials worried that the shot’s limited application and marginal health benefits didn’t justify its inconvenient three-dose course, which had to be completed in a year. Officials were also wary that recommending a personalized, discretionary shot would undermine public trust in vaccines in general.
The CDC ultimately gave a lukewarm recommendation about who should get LYMErix. People at high risk of contracting Lyme “should consider” the vaccine, the agency said, and people exposed to tick-infested habitats, but without frequent or prolonged exposure, “may be considered” for LYMErix shots.
Those squishy definitions sowed confusion among doctors. Many never mentioned LYMErix to patients at all, The New Republic’s “The Politics of Everything” podcast reported, likely limiting the pool of people who might have been interested.
Beset with lawsuits, GSK pulled the vaccine off the market
Even some people who received LYMErix criticized the shot.
“Some people who had been suffering from Lyme for a long time, when the vaccine was out, took it and thought that the vaccine had reactivated the bacteria in their body and created arthritic conditions in them,” Rebecca Onion, who wrote about the downfall of LYMErix, told “The Politics of Everything.”
“This caused them to turn against the vaccine,” she added. “They were an important part of the groups of people who were lobbying for the vaccine to be taken off the market.”

Scientists, however, couldn’t find any evidence that the vaccine caused adverse health problems.
The Vaccine Adverse Event Reporting System — a national monitoring system run by the CDC and FDA — received around 900 reports of adverse LYMErix events between 1998 and 2000. Though US health officials classified 66 reports as serious, CDC and FDA researchers said they didn’t find any unusual side-effect patterns.
But those findings didn’t stop a handful of class-action lawsuits, later consolidated into a single suit, alleging that people had experienced significant adverse reactions to LYMErix. The suit additionally alleged that GSK had concealed evidence about the vaccine’s risk.
As bad press about the vaccines poured in, demand fell from 1.5 million doses in 1999 to a projected 10,000 in 2002, according to the National Institute of Allergy and Infectious Diseases. LYMErix’s anticipated sales also plummeted.
GSK maintained that the vaccine was safe and settled the lawsuit without paying any money to the plaintiffs. But the company was still on the hook for more than $1 million in legal fees.
In early 2002, GSK voluntarily pulled LYMErix off the market.
LYMErix is dead, but Lyme PrEP is giving researchers hope
The quest to prevent Lyme disease isn’t over. A team of researchers at the University of Massachusetts Medical School’s MassBiologics is working on a pre-exposure prophylaxis (PrEP) Lyme disease shot.
The formula is a yearly injection, likely taken at the start of tick season, which would deliver anti-Lyme antibodies to the recipient. It’s different from a vaccine, which triggers the patient’s immune system to make antibodies itself.
Lyme PrEP showed success in studies of mice and primates, and researchers received FDA approval in February to start human trials. If the results are promising, researchers will proceed to larger trials to determine whether Lyme PrEP works. That means Lyme PrEP could reach markets no sooner than 2023.


