What the FDA’s First COVID Vaccine Restriction Means
On May 5, 2022, the Food and Drug Administration (FDA) suddenly announced the limited use of the Johnson & Johnson (Janssen) vaccine, indicating that only people who are not suitable for the Pfizer or Modena mRNA vaccines, or who cannot receive the mRNA vaccines, can receive the Johnson & Johnson vaccine.
What new findings has the FDA made about the side effects of the Janssen vaccine?
First FDA-Restricted Vaccine
This restriction order is different from previous ones.
Usually, “restrictions” are used for “groups unsuitable for vaccination.” For instance, in the United Kingdom, the AstraZeneca (AZ) vaccine is restricted for people under the age of 40, because of its thrombotic side effects and its higher risk for this age group. This restriction is imposed to prevent people with high risk from receiving the vaccine.
However, in the case of the Janssen vaccine, the FDA requires that only people who are not suited for receiving the mRNA vaccines, or who cannot receive the mRNA vaccines, to receive the Johnson & Johnson vaccine.
This is akin to stating that if people cannot get a better vaccine, they can get a worse vaccine. In a slightly exaggerated way, the FDA is saying that if you can’t use a drug with fewer side effects, then you can use a drug with dangerous side effects.
Why would the FDA limit the use of the Johnson & Johnson vaccine this time? Let’s take a look at the entire process and timeline of the Janssen vaccine administration.
A Serious Side Effect of the Janssen Vaccine Was Discovered in 2021
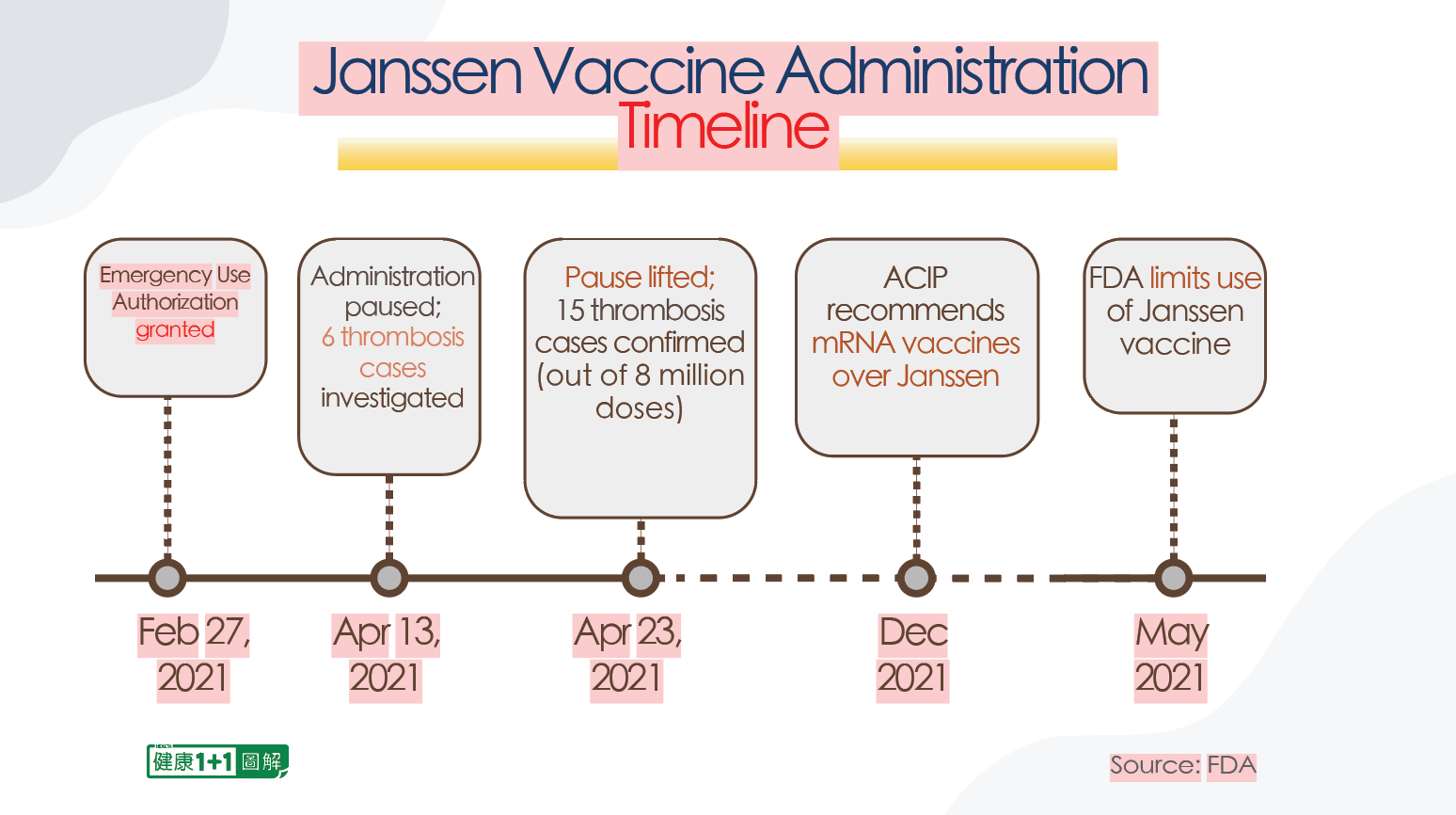
The Janssen vaccine was granted the Emergency Use Authorization (EUA) by the FDA on February 27, 2021.
In March 2021, the side effect of the AstraZeneca vaccine, thrombosis with thrombocytopenia syndrome (TTS), was discovered in Europe.
Immediately afterwards, on April 13, 2021, six cases of the same thrombotic syndrome were found in Janssen vaccine recipients, so the FDA announced a pause in administration of the vaccine and started an investigation.
Ten days later, on April 23, the Janssen vaccine’s administration pause was lifted, with the FDA citing the small number of reported cases and the uncertainty of whether or not some similar cases were caused by the vaccine. On the recommendation of the CDC’s Advisory Committee on Immunization Practices (ACIP), the FDA continued to implement the Janssen vaccine rollout.
However, in December 2021, the CDC’s ACIP issued a cleverly worded recommendation that the Pfizer and Modena vaccines were preferred over the Janssen vaccine. At that time, the ACIP already knew that the Johnson & Johnson vaccine had a serious TTS side effect similar to those of the AstraZeneca vaccine, and that they were life-threatening, but that the numbers were small.
However, neither the ACIP nor the FDA released updated data to the public at that time. They didn’t recommend a pause on the Janssen vaccine’s administration, either. Instead, they only “recommended” the use of Pfizer and Modena vaccines without explaining the reasons to the public.
So, why did it take five months for the FDA to take action, when the ACIP already gave its preferential recommendation in December 2021?
How many people received the Janssen vaccine during those five months? If the FDA had acted in a timely manner, could it have prevented some people from getting serious side effects from the vaccine? These are the questions that we ask about the timing of the FDA’s action.
So, how high is the risk of TTS, a side effect of the Janssen vaccine?

Thrombotic Syndrome’s Mortality Rate Reached 15 Percent
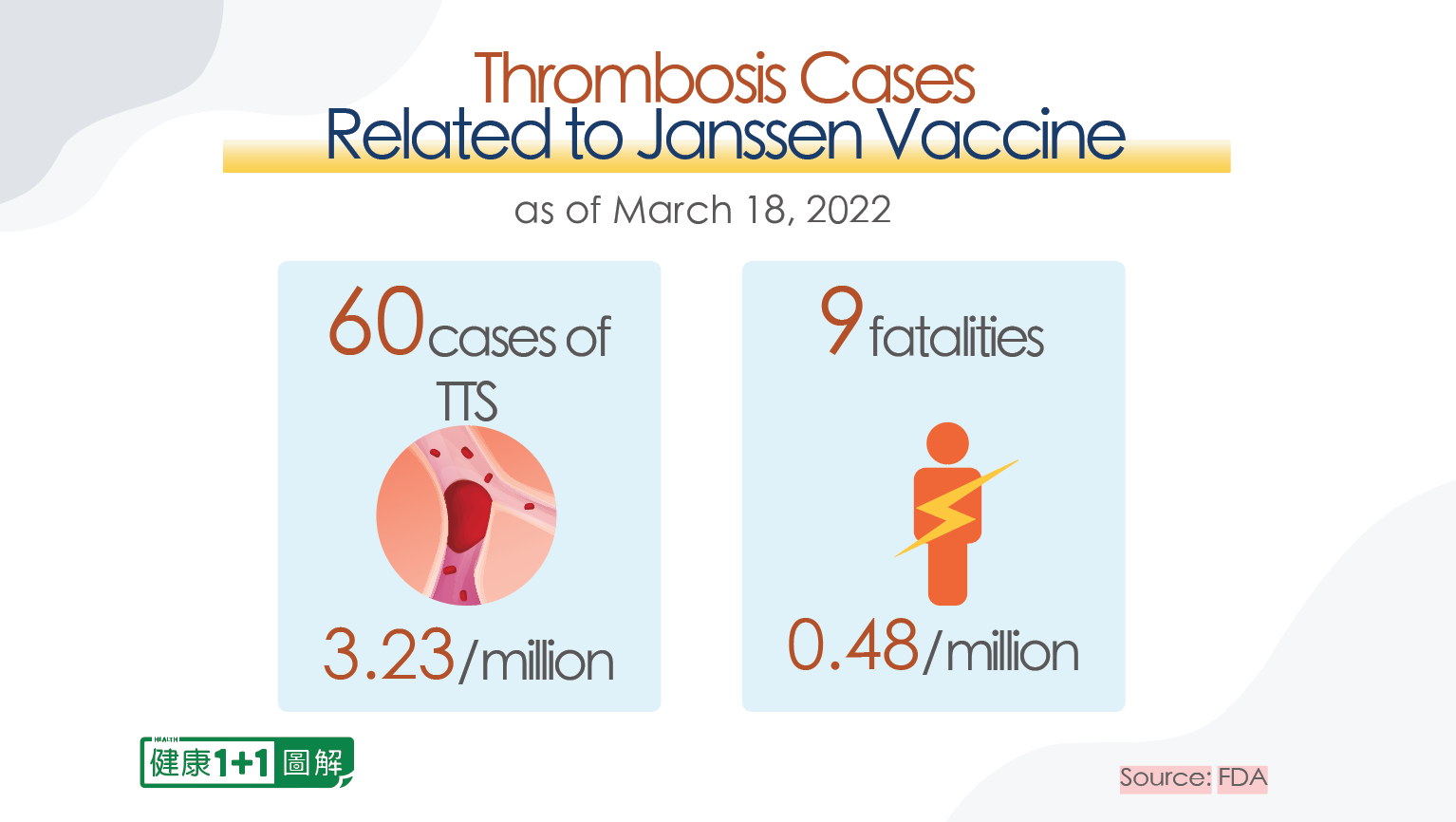
According to the most recent FDA release in May 2022, as of March 18, 2022, a total of 60 cases of TTS had been confirmed in Janssen vaccine recipients. According to the definition from the International Society on Thrombosis and Haemostasis (ISTH), it is actually more appropriately referred to as vaccine-induced immune thrombotic thrombocytopenia (VITT), a term commonly used in Europe. However, the FDA and CDC refer to it as TTS.
The difference between TTS and VITT is that while VITT is clearly thrombotic thrombocytopenia caused by a “vaccine-induced” immune response, the term TTS encompasses symptoms from causes other than the vaccines, thus obscuring the cause of the symptoms.
The latest FDA release mentioned that 60 cases of thrombosis have been confirmed in the Vaccine Adverse Event Reporting System (VAERS), at a rate of 3.23 per million. This ratio may not seem high, and the cases may seem like rare events, but it is a statistical figure passively collected from the VAERS database, and the actual figure would certainly exceed this number.
In addition, the document mentioned nine fatalities out of the 60 thrombosis cases, and the FDA confirmed the fatalities to be vaccine-induced, with a mortality rate of 15 percent.

According to the information collected worldwide on the AZ vaccine, the mortality rate for VITT is approximately 22 percent. Although treatment options are available, many patients’ conditions experience rapid deterioration.
By the end of December 2021, VAERS reported more than 10,000 vaccine-related deaths. In response to these deaths, the FDA and CDC have only explained in general terms that they had no way to medically confirm that these deaths were induced by the vaccines.
Currently, the FDA has confirmed nine fatalities caused by the Janssen vaccine’s side effects, which was a very small number. And experts still believe that the benefits of the Janssen vaccine outweigh its risks. However, how many of the 10,000 plus deaths in the VAERS system were caused by vaccines? And shouldn’t the data be released to the public in a transparent manner?

Deadly Connivance by Regulators
A drug or vaccine causing serious and fatal adverse events is a paramount issue.
The US military was developing a dengue fever vaccine in the 1980s. At that time, the development of the vaccine was at the phase of clinical trials, which had just started. However, deaths related to the vaccine were later discovered.
Although there was no way to completely confirm whether or not the deaths were caused by the vaccine, the vaccine’s development was eventually halted, because the risk of death was taken seriously as one of grave concern.
However, the connivance of pharmaceutical companies and government regulators is now vastly different from before.
In recent years, French vaccine company Sanofi Pasteur developed a dengue vaccine called Dengvaxia. In Southeast Asia, children have been given the vaccine on a massive scale, and there have been many deaths in the Philippines alone. As of August 2019, this vaccine had caused about 600 deaths, but the government still didn’t stop its use.
When a vaccine company’s products pose a serious risk, whether government regulators impose a strict control over them and/or stop their use in a timely manner is an ethical and moral issue.
When the Johnson & Johnson vaccine’s severe side effects emerged, the FDA didn’t ban the vaccine directly, but it instead allowed people who could not receive mRNA vaccines or don’t have access to mRNA vaccines to get the Janssen vaccine. Did they think it would pose no risk to this specific group of people?
Another problem is that beyond the United States, there are many countries around the world still using the Janssen vaccine, and these countries may have spent enormous efforts importing many doses. So what should they do?
In addition, Johnson & Johnson itself hasn’t released any information to respond to the queries, neither explaining nor defending itself. It’s simply leaving it to the media to downplay the issue.
Some people, who have received the Janssen vaccine, may be concerned about its side effects. However, generally speaking, if no symptoms appear more than 20 days after the vaccination, there is usually nothing to worry about.
WHO Recommended Considering a Second Dose of the Janssen Vaccine
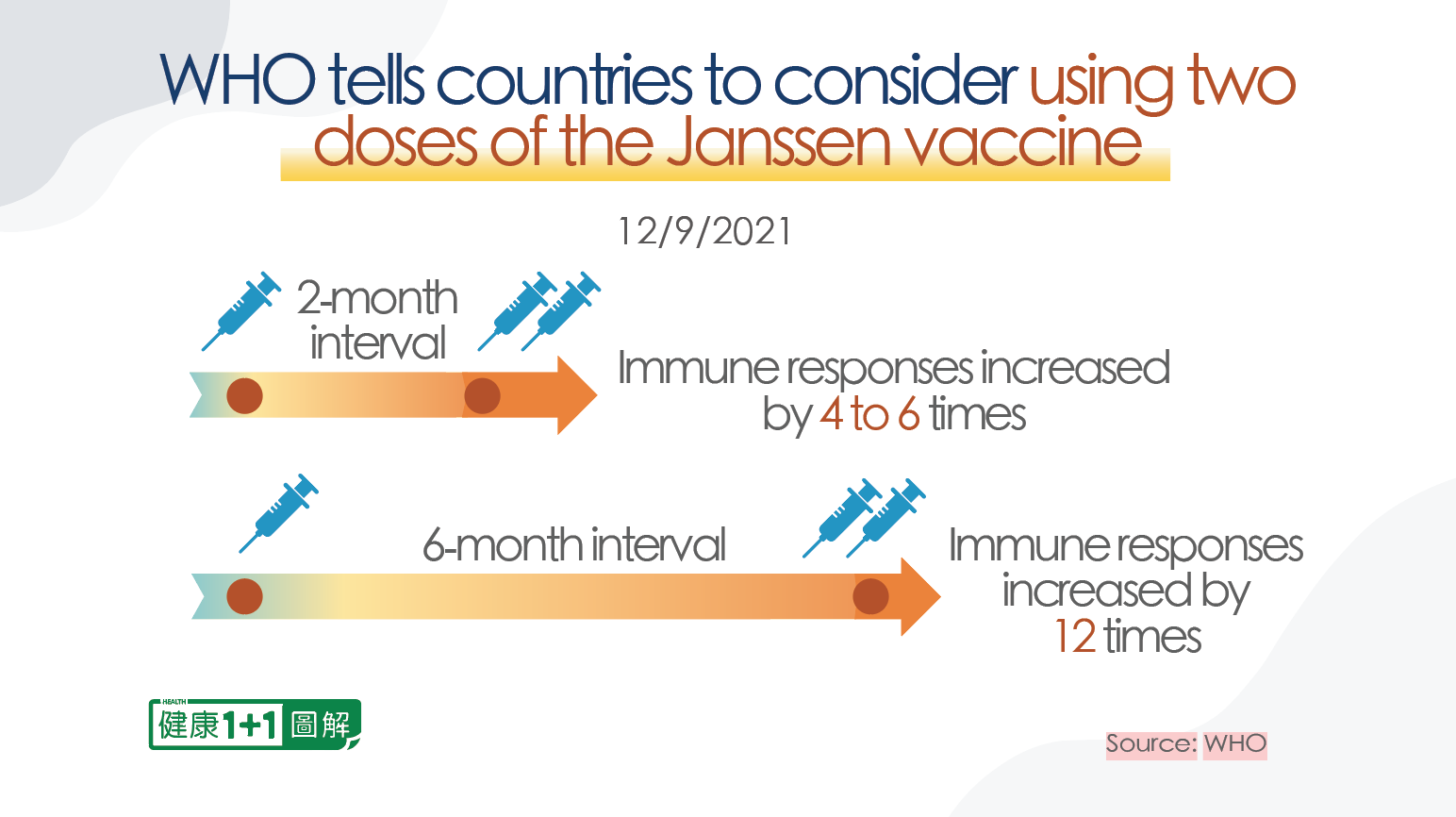
In December 2021, when some European countries had already started restricting the use of the Janssen vaccine, in order to present better data, Johnson & Johnson performed clinical trials of administering two vaccine doses. After two doses of the vaccine were administered at two-month intervals or six-month intervals, changes in the number of antibodies in the body were observed respectively:
- When administered at 2-month intervals, antibodies increased by 4 to 6 times.
- When administered at 6-month intervals, antibodies increased by 12 times.
On December 9, 2021, the WHO recommended countries to consider using two courses of the Janssen vaccine.

The WHO highly values these clinical trial results, but it seems to downplay the issues of adverse events and serious side effects. And it doesn’t emphasize whether or not two doses of the vaccine will increase people’s chances of getting TTS or VITT.
Currently, the WHO’s principal goal is to vaccinate more people across the globe, and to ensure that vaccines are distributed equitably around the world—especially to poor countries. So it hasn’t kept up with the European age restriction on the AZ vaccine.
Of course, there is some merit to this approach. However, authoritative health organizations should also consider the issue of side effects. And when Johnson & Johnson provides clinical trial data, they need to be asked questions about the side effects.
2 Other Serious Side Effects of the Janssen Vaccine
Data collected by the WHO from around the world indicate that the Janssen vaccine has two other serious side effects, in addition to the TTS.
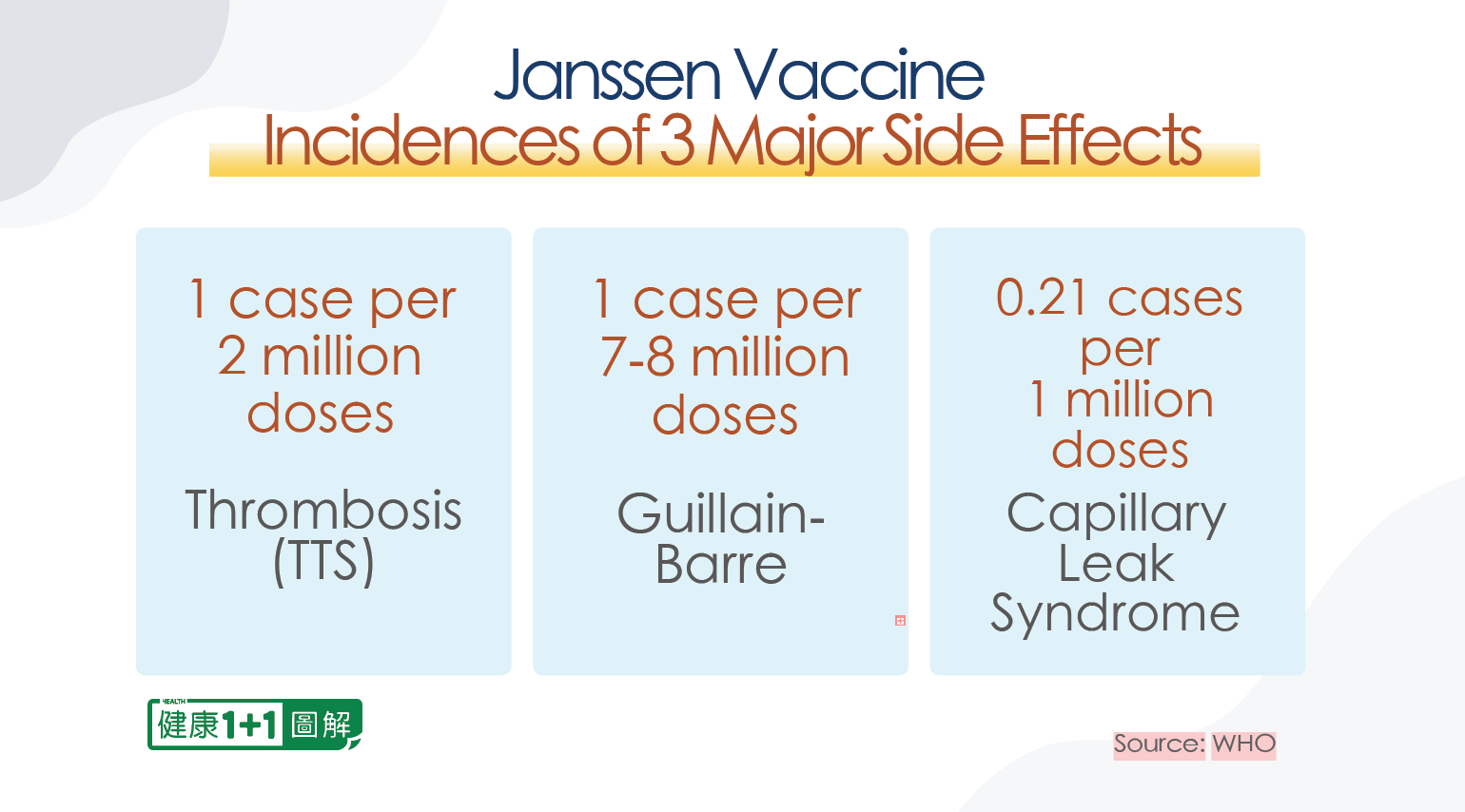
One of them is the Guillain-Barre Syndrome (GBS), which refers to the immune system attacking its own nervous system, with an incidence of one in 7 to 8 million.
The other is the capillary leak syndrome (CLS), in which plasma leaks out of blood vessels and infiltrates into tissues, causing a generalized edema, with an incidence of 0.21 per 1 million and a relatively high mortality rate.
Although the number of cases found is still relatively small (this is also due to the statistical methods used), these two serious side effects are still worthy of attention.
Nowadays, people are used to pharmaceutical companies writing their side effects in fine print, not easily noticed by the public. Drug companies need to consider their own interests, but then the regulators should warn the public about such side effects, rather than just recommending certain drugs while downplaying their serious side effects.
References
https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-limits-use-janssen-covid-19-vaccine-certain-individuals
https://www.healio.com/news/womens-health-ob-gyn/20211216/acip-approves-statement-preferring-mrna-covid19-vaccines-over-the-johnson-johnson-vaccine
https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-limits-use-janssen-covid-19-vaccine-certain-individuals
https://www.cnbc.com/2021/08/12/blood-clots-linked-to-astrazeneca-shot-have-22percent-mortality-rate-study.html
https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE-recommendation-Ad26.COV2.S-GRADE-ETR-annexes
Views expressed in this article are the opinions of the author and do not necessarily reflect the views of The Epoch Times.

