Fact Check: COVID Vaccine Trials Did NOT Skip Animal Testing Because All The Animals Were Dying — No Steps Were Skipped

Did COVID vaccine trials skip animal testing and go straight to people because all the animals were dying? No, that’s not true: In an email to Lead Stories, the U.S. Food and Drug Administration (FDA) said, “The claim is completely false. All authorized and approved COVID-19 vaccines underwent animal studies.”
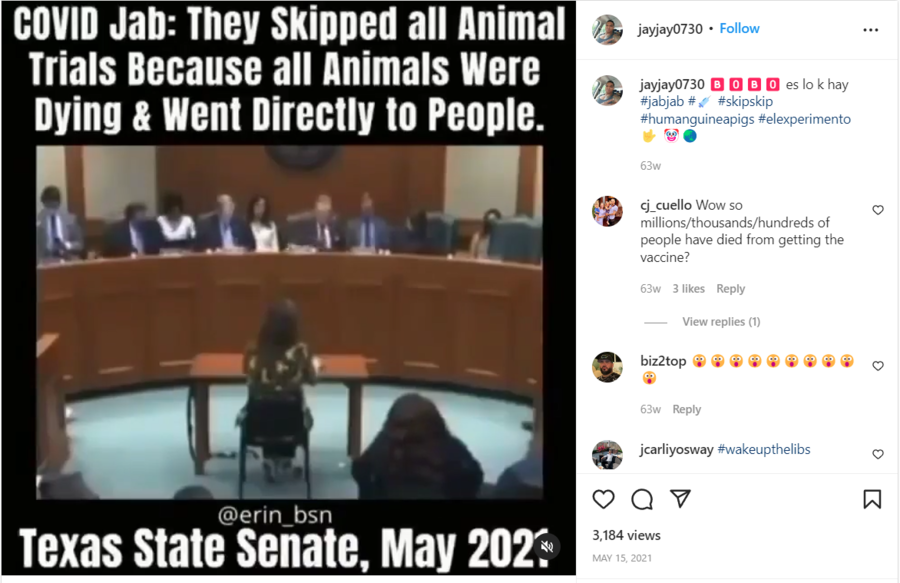
The claim appeared in an Instagram post and video on May 15, 2021, under the title “🅱️🅾️🅱️🅾️ es lo k hay [Fool, it is what it is] #jabjab #💉 #skipskip #humanguineapigs #elexperimento🤟 🤡🌏.” It opened:
COVID Jab: They Skipped all Animal Trials Because all Animals Were Dying & Went Directly to People. Texas State Senate, May 2021
This is what the post looked like on Instagram at the time of writing:
(Source: Instagram screenshot taken on Wed Aug 3 18:17:44 2022 UTC)
The video in the post is from a Texas Senate Committee on State Affairs hearing from May 6, 2021. The clip features state Sen. Bob Hall questioning pediatrician Angelina Farella about COVID-19 vaccines. The following is a transcript of the back-and-forth between the pair, starting at 44:24 in the original video:
Hall: And have you seen any other vaccine that was put out for the public that skipped the animal test?
Farella: Never before, especially for children.
Hall: And what I have read, they actually started the animal tests and, because the animals were dying, they stopped the tests.
Farella: Correct.
Hall goes on to say that the American citizens are now the guinea pigs. The state senator is not a physician and has been cited by Texas media for spreading COVID treatment and vaccine misinformation.
In an August 3, 2022, email, FDA Press Officer Veronika Pfaeffle told Lead Stories there’s no truth to what Hall is saying:
The claim is completely false. All authorized and approved COVID-19 vaccines underwent animal studies. These studies are referenced in the Pfizer-BioNTech, Moderna and Janssen EUA decision memos and in the Summary Basis of Regulatory Action documents for Comirnaty and for Spikevax.
Additional resources on authorization and approval:
- https://www.fda.gov/media/151716/download
- COVID-19 Vaccines
- Development and Licensure of Vaccines to Prevent COVID-19
- Emergency Use Authorization for Vaccines to Prevent COVID-19 (this guidance contains an appendix titled: APPENDIX 2: EVALUATION OF VACCINES TO ADDRESS EMERGING SARS-COV-2 VARIANTS”)
When the trials were taking place, Pfizer, Moderna and Johnson & Johnson all disclosed the results of their trials on non-human subjects in official statements from 2020.
The vaccine makers
In a separate August 3, 2022, email, Kit Longley with Pfizer Global Media Relations said:
We can confirm we conducted and completed pre-clinical studies in 2020, which are required by FDA and other regulatory bodies.
You can find more information on our pre-clinical studies here:
Lead Stories also reached out to Moderna and Johnson & Johnson but had not received a response at the time of writing.