Fact Check: FDA Did NOT Report Pfizer COVID-19 Vaccine ‘Causes Blood Clots’

Did the Food and Drug Administration report that Pfizer-BioNTech COVID-19 vaccines “cause” blood clots? No, that’s not true: A study conducted in part by the Food and Drug Administration (FDA) identified four new statistical signals for “modestly elevated risks” of certain conditions, including blood clots, for people over 65. However, the study authors specified that the findings “are not necessarily causal and may be due to factors potentially unrelated to vaccination.” They emphasized that “more robust epidemiologic studies” are needed.
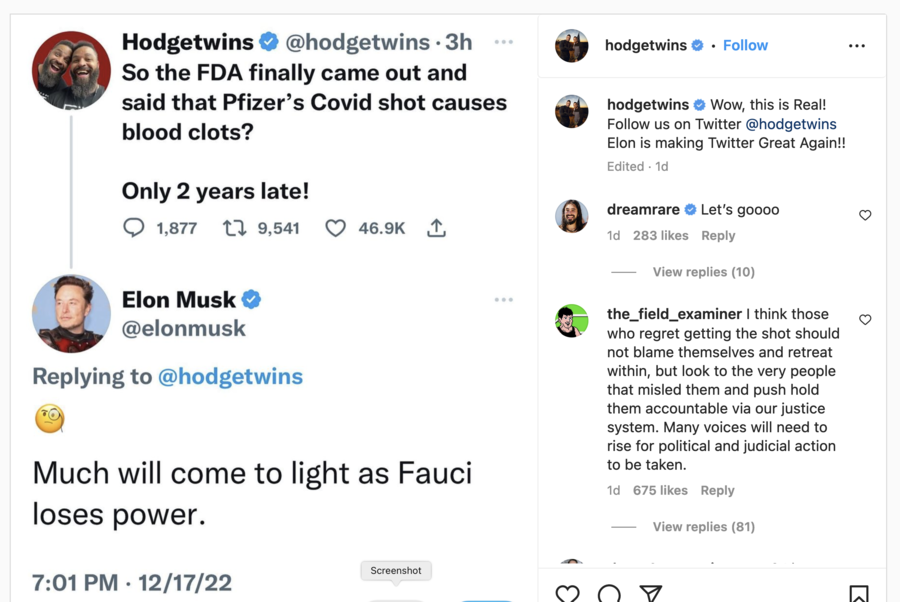
The claim that “Pfizer’s Covid shot” causes blood clots was made in a December 17, 2022, tweet (archived here) by @Hodgetwins, a standup comedy act comprised of twin brothers Keith and Kevin Hodge. Twitter CEO Elon Musk responded to the tweet, amplifying its visibility.
The Hodge brothers claimed that the FDA “finally came out and said that Pfizer’s Covid shot causes blood clots.” Screenshots of the tweet with Musk’s response were then reshared on Instagram. The posts read:
[@Hodgtwins]: So the FDA finally came out and said that Pfizer’s Covid shot causes blood clots? Only 2 years late!
[@elonmusk]: Much will come to light as Fauci loses power.
Here is how the post appeared at the time of writing:
(Source: Instagram screenshot taken Mon Dec 19, 11:09:08 2022 UTC)
This claim appears to have originated in an article published by The Epoch Times on December 17, 2022, (archived here) that cited a December 1, 2022, study published in the peer-reviewed journal Vaccine (archived here). The title of the study is, “Surveillance of COVID-19 vaccine safety among elderly persons aged 65 years and older.” Its researchers represented the FDA, health care policy research center Acumen LLC, Stanford University’s Department of Economics and the Centers for Medicare & Medicaid Services, a federal agency that manages publicly funded health care.
Citing the study, The Epoch Times reported that “Pfizer’s COVID-19 vaccine has been linked to blood clotting in older individuals, according to the U.S. Food and Drug Administration (FDA).” The article read, in part:
FDA researchers, crunching data from a database of elderly persons in the United States, found that pulmonary embolism–blood clotting … in the lungs–met the initial threshold for a statistical signal and continued meeting the criteria after a more in-depth evaluation. …
The FDA said it wasn’t taking any action on the results because they don’t prove the vaccines cause any of the four outcomes, and because the findings ‘are still under investigation and require more robust study.’
However, the FDA did not report that the Pfizer-BioNTech COVID vaccine caused — or was even linked to — blood clots. In fact, the authors of the cited study pointed out the lack of evidence for a causal link between the two, writing:
The statistical signals of four serious outcomes are not necessarily causal and may be due to factors potentially unrelated to vaccination. Additional analyses indicated that the potential association was less than twice the historical rates and may be associated with factors not accounted for in the near real-time surveillance methods.
As part of the study, researchers analyzed the Medicare health insurance data of more than 25 million people 65 and older who had received a COVID vaccine between December 11, 2020, and January 15, 2022.
The team identified four new “statistical signals,” indicators of potential connections not yet established, for “modestly elevated risks” that include the development of blood clots. The National Library of Medicine describes statistical signals as a way to detect previously unknown causal associations between medicines and unexpected events.
These associations can serve as an “early warning system” for health problems. The researchers note that such surveillance measures have been used to characterize the safety of the annual influenza vaccine.
For the Pfizer-BioNTech COVID vaccine, the researchers detected statistical signals for the following four serious outcomes for a restricted group of individuals over 65:
-
Myocardial infarction (AMI) — also known as a heart attack;
-
Pulmonary embolism (PE) — a blood clot that moves into the lungs from a blood vessel elsewhere;
-
Disseminated intravascular coagulation (DIC) — abnormal blood clotting;
-
Idiopathic thrombocytopenic purpura (ITP) — low levels of blood platelets, which stop bleeding
The researchers wrote, however, that these findings do “not prove that vaccines cause the safety outcomes,” nor do they “establish a causal association between the outcomes and vaccination.”
That’s because the study looked at medical billing codes for certain conditions among people 65 and older who had received the COVID vaccine. It did not account for underlying risk factors such as pre-existing multiple diseases in a vaccine recipient.
Also, since the researchers were referring to diagnosis billing codes in Medicare and Medicaid claims, the data may “underestimate or overestimate certain clinical conditions because of reimbursement priorities.”
Additionally, the study authors add that the findings may not “be generalized to those younger than 65 years and adults who are uninsured or received only commercial health insurance.”
To compensate for these shortcomings in the study, its authors intend to run “further epidemiological studies along with [a] medical record review …”
The researchers cautioned that:
Our new findings of statistical signals for four important outcomes for the BNT162b2 [Pfizer-BioNTech] vaccine should be interpreted cautiously because the early warning system does not prove that vaccines cause the safety outcomes.
FDA strongly believes the potential benefits of COVID-19 vaccination outweigh the potential risks of COVID-19 infection. Per FDA communication of these findings, FDA is currently not taking any regulatory actions based on these signal detection activities because these signals are still under investigation and require more robust study.
Lead Stories contacted the FDA and Acumen LLC for official statements and will update this fact check accordingly if responses are received.
Additional fact checks related to COVID-19 vaccines can be found here.