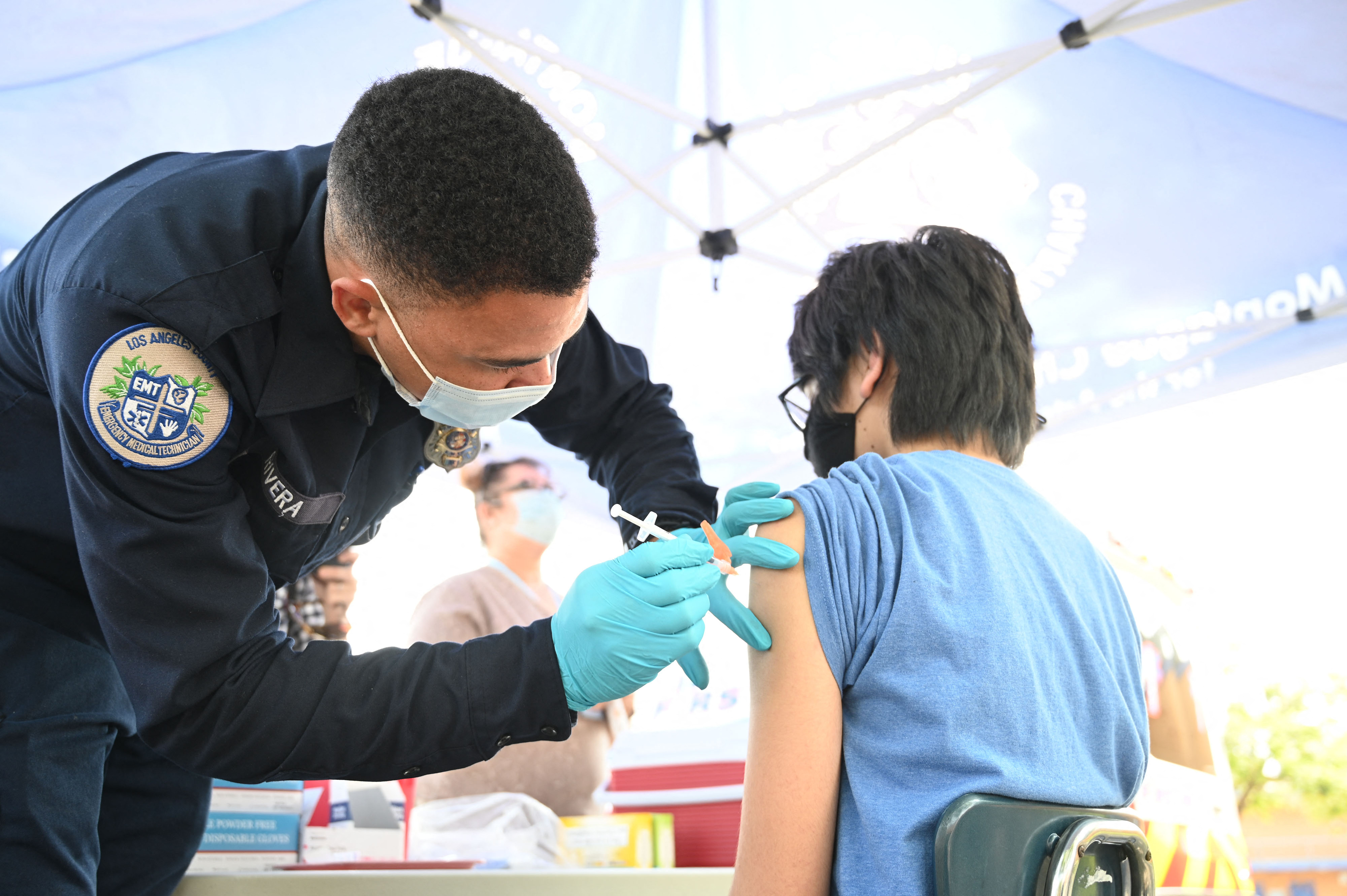
New Study Aims to Learn More About COVID-19 Vaccine Injuries
Researchers are estimating thousands of people will participate.
Researchers who hope to learn more about COVID-19 vaccine injuries have launched a new study.
“The primary goal of this project is to document the symptoms and treatments of vaccine-injured individuals. With enough participants, we hope to identify symptom patterns or clusters, and treatments that align with these symptom patterns or clusters,” Linda Wastila, director of research at the University of Maryland’s Peter Lamy Center on Drug Therapy and Aging, told The Epoch Times in an email.
“One of the most frustrating aspects of COVID-19 vaccine injury is the lack of medical and health acknowledgement, and the lack of knowledge of how to manage and treat symptoms,” she added.
Ms. Wastila, who holds a bachelor’s degree of science in pharmacy, a master’s degree of science in public health, and a doctorate in health policy, is leading the study, with assistance from the vaccine-injured support group React19.
They will be asked about their health before receiving a COVID-19 vaccine and symptoms they experienced after vaccination. They will also be asked about tests they underwent, diagnoses they received, and treatments that were administered.
Participants must be 18 years of age or older.
“We hope to use this data to publish patient-driven data about vaccine reactions in hopes of educating the medical community and other vaccine-injured individuals,” React19 said on its website.
React19 says it plans to publish the results in top research journals.
No Funding
The study is not receiving any funding; instead, it is being carried out by Ms. Wastila and React19 volunteers in their spare time.
Ms. Wastila said that she did not think such a study would receive funding from federal or state governments, and that it would be difficult and time-consuming to obtain funding from private sponsors.
“To garner funding also [would] take considerable time and effort, and the risks of not receiving funding are high. This survey is ‘urgent’ and we don’t have a year or longer to find out if our research proposals are funded,” Ms. Wastila said.
The study was approved by an Institutional Review Board, according to React19.
Researchers are estimating thousands of people will fill out the study. Some 995,000 reports of adverse events after COVID-19 vaccination have been lodged with the U.S. vaccine injury database as of Oct. 27.
“The data we receive will represent the most comprehensive and complete record of COVID-19 vaccine injured to date,” Ms. Wastila said.
React19 said that early returns have shown “interesting patterns” but encouraged more people to participate.
“You do not have to take the survey in one sitting. You can start the survey and save your progress so that you can continue when you prefer,” React19 said.
Researchers are relying on respondents to answer the questions truthfully. They will not be attempting to verify the information provided.
Spur More Research?
The findings from the study may be able to identify treatments that have been successful.
“Our hope is that we will be able to aggregate symptoms with treatments, and ideally identify patterns of treatments most helpful in alleviating symptoms and improving health and quality of life,” Ms. Wastila said.
She said that the survey approach was taken “because there are NO available data—from other surveys, from administrative claims, from registries—that adequately acknowledge and/or account for these symptoms and treatments.”
The survey, React19 says, could spur additional research.
“We hope findings provide preliminary data for future funding to allow larger surveys and to provide direction for analyses using larger, already-collected data (such as health insurance data),” React19 said.