The FDA Can Now Withhold COVID Vaccine Safety Records. Again, We Ask – Are The FDA, With Their Ties to Bill Gates, Fit For Purpose?

Under a recent order from a federal judge, the U.S. Food and Drug Administration (FDA) is being allowed to withhold COVID-19 vaccine safety data for at least another six months due to meeting the criteria of “exceptional circumstances.” Lawyers representing the FDA have said that the agency is “overburdened by court orders” forcing it to produce a certain amount of records pertaining to the authorisation of the Pfizer and Moderna COVID-19 vaccines.
The Epoch Times reported that the FDA’s Center for Biologics Evaluation and Research is “dealing with an unprecedented workload” and “specific and unprecedented hardships,” the lawyers said in a motion for a stay. They asked for an 18-month pause in processing a Freedom of Information Act request that seeks the results of COVID-19 vaccine safety data mining.
The nonprofit Informed Consent Action Network sued after the FDA declined to provide any of the materials. The agency also refused to provide them to The Epoch Times. Companion analyses that were obtained by The Epoch Times showed U.S. authorities detected hundreds of safety signals for the COVID-19 vaccines, many of which authorities have never provided evidence of examining further. Authorities said the analyses the FDA ran were “more robust.”
U.S. District Judge Reggie Walton sided with the government, “Unfortunately I would have to conclude that because of the extraordinary orders that have been issued by the courts down in Texas … requiring just an exorbitant amount of document production within a relatively short period of time, considering the number of documents involved and all of the other litigation I know that agencies have, I would have to conclude that extraordinary circumstances have been established,” Judge Walton said in a Nov. 20 hearing in Washington, according to a transcript reviewed by The Epoch Times.
The ruling means the FDA can continue withholding materials on its analyses of reports to the Vaccine Adverse Event Reporting System, which have spiked since the introduction of COVID-19 vaccines.

The Food and Drug Administration
The FDA is a licensing agency within the U.S. Department of Health and Human Services. However, they are tasked with the job of assuring the quality and safety of vaccines as well as the food supply and human and veterinary drugs among other products. Source
They have the monopoly in their field as a licencing agency and without their approval, it is illegal to offer a product for sale or use among society.
What this means is they have the final say regardless of whether they have made errors in their assessments and approvals. It is obvious to many of us that the FDA approved COVID vaccinations and ignored not only the lack of necessity or efficacy but also their safety and they should be made to show all of the data that allowed them to make such a reckless decision to approve them. Source
The Freedom of Information Act
The Freedom of Information Act (FOIA) requires agencies to respond to FOIA requests in certain timeframes but agencies can skirt the requirements if they show that “exceptional circumstances” exist and they are conducting “due diligence, according to the Epoch Times.
They report that two suits brought against the FDA for not adhering to timelines in FOIA requests for documents relating to the agency’s authorizations of the COVID-19 vaccines yielded rulings in favor of plaintiffs. The court in those cases ordered the FDA to produce a certain amount of documents responsive to the requests on a monthly basis.
Those orders required the agency to produce at least 90,000 pages per month starting in July, though the FDA has since finished production in one of the cases. Those orders, coupled with an increase in FOIA requests and lawsuits in recent years, meet the criteria of exceptional circumstances, government lawyers said. They said most FDA FOIA workers are tied up with the orders.
FOIA Request Were Rising Before 2020
Lawyers representing the plaintiff said the FDA had not met the criteria of exceptional circumstances, noting that FOIA requests have been on the rise since before the COVID-19 pandemic.
“As the law currently stands, in order to show exceptional circumstances, it must be for the relevant time period. And the FDA’s papers have shown that they’ve known about this uptick and have been able to see it happening since 2019,” Elizabeth Brehm, one of the lawyers, said during the recent hearing.
“The FDA also did not exercise due diligence because it initially denied the FOIA request outright, and failed to process an appeal in the time proscribed by the law, which prompted the lawsuit,” she added.
The Epoch Times writes that the FDA has since done some work on the request and has identified 150 responsive records, but, said more time was needed to review those records and make redactions, as well as conduct additional searches that could turn up additional relevant materials.
The Lawyers also countered that the FDA did not slow down COVID-19 vaccine authorisation due to a lack of resources and that it should apply the same focus to processing FOIA requests.
It is true that the COVID jabs were authorised speedily, and the FDA were the agency to have the final say in their approval.
I kept receipts, I guess you could say, in a piece I published here in The Expose ON October 2021 which shows that the FDA had approved the COVID Jab despite many reasons not to, and they cannot deny that they knew about them.
Are the FDA Fit for Purpose? Dangerous lies and Bill Gates Ties

Pfizer and BioNTech (the sponsor) submitted an EUA request to the FDA on November 2020, for an “investigational COVID-19 vaccine (BNT162b2) intended to prevent the unproven to exist, COVID-19” the Pharma company provided data in order to aid the approval which was intended to imply that the vaccine would protect individuals more effectively than prior infection of the virus.
It seems clear that the FDA approval was granted despite either the FDA fully analysing the data and/or ignoring the red flags which would have deemed them unsafe and ineffective and the products should never have been authorised. Source
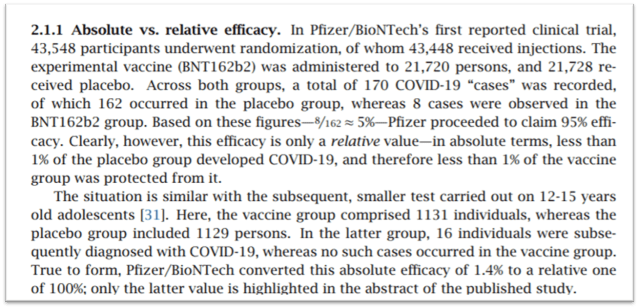
Pfizer’s Play on Numbers
For example, Pfizer’s data reported 8 cases of what they deemed to be “COVID-19” among vaccinated people which amounted to an incidence of 0.044%, however, they also reported 7 cases among unvaccinated people who had initially tested positive.
This group was considerably smaller, and the 7 cases were to translate into a ninefold higher incidence (0.38%). Despite advice that vaccines cannot surpass the immunity to be gained from natural infection and also a study conducted in Dec 2020 that found individuals who they deemed to have previously been diagnosed with the “SARS-CoV-2 infection” were, in fact, unlikely to benefit from COVID-19 vaccination. Source
Nevertheless the FDA ignored the evidence, the common sense, the study, and also Pfizer’s obvious play with numbers.
Exaggerated Efficacy
The FDA also appeared to have ignored the figures to show apparent efficacy that Pfizer/BioNTech touted, that were simply not true.
Pfizer converted the absolute efficacy of 1.4% to a relative one of 100%, see Figure below.
This fact was picked up by lawyer Renate Holzeisen who devised a statement that he has submitted to the European General Court together with a lawsuit to challenge the use of the mRNA vaccine on children over the age of 12.
Holzeisen stated that Pfizer had also made unlikely claims and contradictions in their evidence on efficacy, which he says “were reported very modestly when expressed in absolute terms, yet even this low efficacy cannot be accepted at face value,” this was also apparent from the assessment reports prepared by the FDA.
The efficacy of the Pfizer mRNA vaccine, therefore, was known to be exaggerated at best very early on, yet again this was another area that the FDA chose to ignore. Source
Adverse Events
The FDA continued to ignore evidence that should have halted the administration of the jab, including findings that were arrived at by esteemed independent scientists and researchers in the fields of immunology and microbiology since early 2021.
The experts had been warning medical regulators of vaccine related blood clotting, bleeding and blood abnormalities. These were findings that were based on established immunological science which predated the roll out of vaccines around the world.
Just a few months following the jab rollout it was clear that the benefits were highly doubtful, one study alone by the Doctors for COVID Ethics found that the harm caused by the vaccine was very well substantiated as already by July 2021, there were more than 15.000 vaccination-associated deaths documented in the EU drug adverse events database (EudraVigilance), and over 7.000 more deaths within the UK and the US. Source.

Yet again, Despite the urgent and obvious dangers of the vaccinations yes you guessed it, the FDA ignored this too and the FDA were willing to allow lives to be lost, when even in their own admission they knew that “additional evaluations including data from clinical trials and from vaccine use post-authorisation will be needed to assess the effect of the vaccine in preventing either virus shedding or transmission.
In other words, there was no evidence that transmission of this alleged virus would be reduced, There were no trials even designed to evaluate such an effect and any clinical trials carried out by Pfizer on all tested age groups contained no proof of any benefits from the vaccine. Source
So, why did the FDA approve the Jab?
Links With Gates and Conflicts of Interest
Conflicts of interest may have got in the way of rational decisions concerning the safety of the public, it could be argued.
For instance:
- The previous head of the FDA Scott Gottlieb, was appointed to the board of directors of Pfizer just three months after resigning from his position.
- Another member of Pfizer’s Board of Directors, Dr Susan Desmond-Hellmann, headed the Bill and Melinda Gates Foundation until 2020.
- Professor Holly Janes, a co designer of the trials for both Pfizer and Moderna mRNA for Fauci’s NIAID from her Seattle center, which is also funded by the Gates Foundation is also a member of the FDA Vaccine Committee until 2023. Source

There were other conflicts of interest and links within the FDA to the Gates Foundation which must be considered to have been influential to the FDA when they have made such a reckless decision to have approved and continued to approve such a dangerous product.
Get Staffed.
They cannot now show how they did the math. In support of the FDA, however, Steven Levy, a lawyer representing the FDA, said that the agency has made “incredible efforts” to hire more people to process FOIAs following the 2022 court order forcing it to produce information on Pfizer’s COVID-19 vaccine.
The FDA had nine staff and one branch chief at the time and has hired 10 contractors since. This spring, it received approval to hire six more full-time workers. The hiring didn’t start until after the order because it was “an extraordinary unforeseen order,” Mr. Levy said.
Judge Walton, appointed under President George H. W. Bush, said that Congress was unlikely to approve more money for the FDA and expressed frustration at how he has multiple FOIA cases involving thousands of documents that need to be processed. He claimed the FDA, which received $8.4 billion from Congress for fiscal year 2023, has “limited resources.”
“To a large degree we have a dysfunctional government that doesn’t have, it seems, the capacity to address some of these issues related to legislation that Congress has passed,” Judge Walton said. The government “has, in fact, made an effort to increase its capacity to process documents where requests are made to this agency pursuant to FOIA, and realistically I have no reason to believe that greater efforts would have resulted in greater resources,” he added. That’s when he entered the order in favor of the FDA.
Ms. Brehm declined to comment. Judge Walton said he hoped circumstances change in the future, so he’ll review the case every six months. He scheduled a conference in May 2024. The case is Informed Consent Action Network v. Food and Drug Administration, in the U.S. District Court for the District of Washington. Source.
Who Will Assure The Quality of The FDA?
Clearly, the records pertaining to the authorisation of the Pfizer and Moderna COVID-19 vaccines should have available to have been produced at the beginning of the worldwide rollout, disclosure would have undoubtedly prevented so many adverse events and deaths.
However, the new ruling means the FDA can continue withholding vaccine safety data and materials on its analyses of reports to the Vaccine Adverse Event Reporting System, which have spiked since the introduction of COVID-19 vaccines, disgracefully being free to “skirt their requirements for FOIA” for at least another six months.
What we can ascertain from all of this, is, the Food and Drug Administration does not have the interest of the people at their core, they have shown, as many others have over these plandemic years, that they too, put wealth over the health of the worldwide population.
We cannot trust the Food and Drug Administration to do the job that they are tasked to do in assuring the quality and safety of these mRNA vaccines or any other product for that matter. But who can we rely on to be responsible for assuring the quality of the FDA?
They are clearly not fit for any other purpose than to aid the cult agenda.
This article has been archived for your research. The original version from The Exposé can be found here.



