Fact Check: Red Cross Blood Donation Question Does NOT Prove COVID-19 Vaccine Is Harmful
Does a Red Cross blood donation question prove that COVID-19 vaccines are harmful? No, that’s not true: The Food and Drug Administration requires the question of all blood collectors in the United States. The federal agency allows people to donate blood immediately after getting a COVID vaccine if they feel well and have no symptoms, as long as the vaccine is approved for use in the United States by the agency.
The claim appeared in a post (archived here) published on X (formerly Twitter) by DC_Draino on February 20, 2024. The post’s caption said:
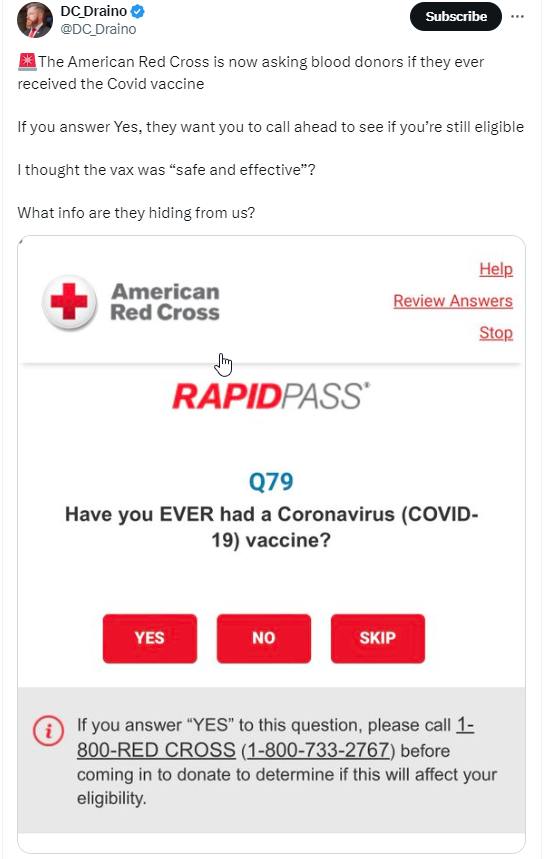
🚨The American Red Cross is now asking blood donors if they ever received the Covid vaccine
If you answer Yes, they want you to call ahead to see if you’re still eligible
I thought the vax was ‘safe and effective’?
What info are they hiding from us?
This is what the post looked like on X at the time of writing:
(Source: X screenshot taken on Wed Feb 21 15:59:45 2024 UTC)
The question
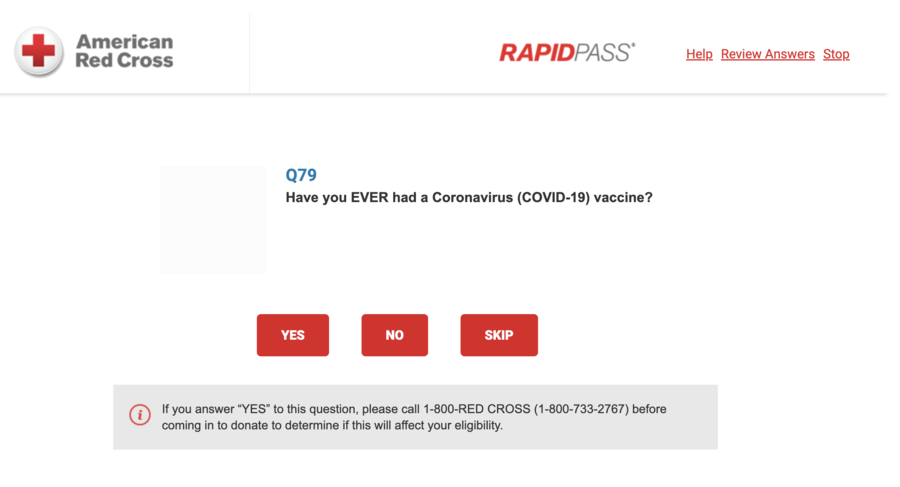
The social media post suggests COVID vaccines are not “safe and effective,” because the Red Cross RapidPass online questionnaire requires a phone call to the organization to determine eligibility if the person has ever had a coronavirus vaccine. A screenshot of the question on the Red Cross website appears below:
(Source: Red Cross website screenshot taken on Wed Feb 21 17:05:01 2024 UTC)
The answer
Red Cross spokesman Daniel Parra told Lead Stories in a February 21, 2024, email that the Red Cross, along with all other blood collection organizations in the United States, must follow eligibility criteria established by the Food and Drug Administration (FDA). This includes guidelines concerning the eligibility of blood donors who have received vaccines, such as the COVID vaccine, among others. He added:
The FDA permits individuals to donate blood with no wait period after receiving a COVID-19 vaccine as long as they are feeling well and symptom-free, and the vaccine they received is one approved by the FDA for use in the US. Those who report they have received a COVID-19 vaccine are asked to provide the name of the manufacturer to ensure it is an FDA-approved vaccine. If the donor cannot remember the name of the manufacturer, they are asked to wait two weeks from their vaccination to give blood.
For some vaccinations, the FDA requires varying wait times to donate blood depending on the vaccine. This includes wait times that can vary from 2 to 4 weeks for a number of vaccines including but not limited to measles, mumps and rubella, chicken pox, shingles, polio, yellow fever, hepatitis B, and others. Due to this, the Red Cross and all blood collectors ask each potential donor about a history of vaccination to determine eligibility.
More information about donor eligibility is available under the “Immunization, Vaccination” tab on the Red Cross website.
Additionally, Parra said:
It’s important to point out donations from those who have been vaccinated for COVID-19 are safe for transfusion. Similar to other vaccines such as measles, mumps, or influenza, the COVID-19 vaccine is designed to generate an immune response to help protect an individual from illness, but vaccine components themselves are not found within the bloodstream.
Additionally, a donor’s immune response is not impacted by giving blood. Donating blood after receiving a COVID-19 vaccine does not reduce a donor’s protection from the virus.
Read more
Additional Lead Stories fact checks of claims about vaccines can be found here.