Unauthorized Changes to COVID-19 Test Formulas Trigger FDA Warning Against Cue Health
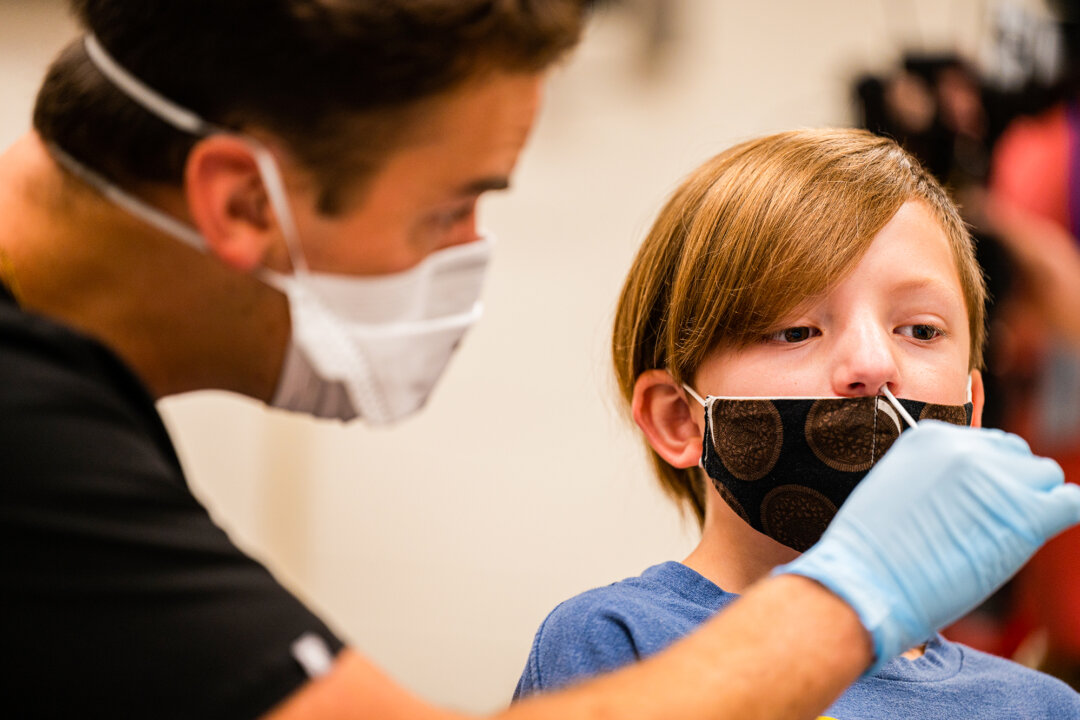
The U.S. Food and Drug Administration (FDA) is warning people to avoid using over-the-counter Cue Health’s COVID-19 tests due to an increased risk of giving false positive results.
The federal agency suggests that any consumers who may have Cue Health brand tests should dispose of those tests and consider using different COVID-19 tests that have been authorized by the FDA.
However, the FDA noted, if testing was performed more than two weeks ago, there is no reason to suspect a person is currently infected with the virus.
Changes Without Approval
Cue Health’s brand tests differ from typical PCR testing. The company relies on molecular testing to detect a virus’s genetic material. Similar to typical PCR tests, the Cue COVID-19 test requires a lower nasal swab. The swab is placed onto a cartridge reader, which is then inserted into a small, at-home lab. The results are delivered to the user’s smartphone within 20 minutes.
However, according to the FDA, Cue Health made several changes to the tests without the federal agency’s approval.
The COVID-19 tests were originally approved under the FDA’s emergency use authorization conditions in June 2020 for point-of-care settings. Cue Health’s COVID-19 test received a second emergency use authorization in March 2021 for consumer use at home.
In the letter, the FDA wrote Cue Health changed the formula of the substrate used to detect the virus. Other changes included updating the device firmware of the test to add functionality to detect failure and modifying the test enough to change the raw electrochemical signal from the reaction during the test.
“These are significant modifications, as they have a strong likelihood to impact the performance of the device due to significant changes to reagent formulations and device design of these devices,” according to the Center for Devices and Radiological Health, an FDA branch charged with making sure patients and providers have access to safe and effective products.
The FDA barred Cue Health from distributing the tests until receiving new authorization.