1st mRNA Cancer Jab Given in UK Trial
Although described as a vaccine, the BioNTech-developed therapies do not confer immunity but are said to target remaining cancer cells after surgery.
A man with bowel cancer has become the first person in the UK to receive a personalised mRNA therapy developed using the same technology used to make the Pfizer-BioNTech and Moderna COVID-19 jabs.
Although described by the National Health Service (NHS) as “vaccines,” the jabs are not designed to prevent cancer cells from developing and are being trialled exclusively in cancer patients.
They are made by taking a sample of a patient’s tumour—removed during surgery—followed by DNA sequencing, and in some cases the use of artificial intelligence. A personalised anti-cancer jab specific to each patient’s tumour is then created in the lab.
Scientists claim the jabs can stimulate a patient’s immune system to recognise and attack any cancer cells that remain after surgery, which in theory could stop the disease from recurring.
Last year, the UK government signed an agreement with German company BioNTech to provide up to 10,000 patients with precision cancer treatments by 2030. The jabs being trialled have been jointly developed by BioNTech and Genentech, a member of the Roche Group, and have not yet been approved by the regulator.
Scientists Have Linked mRNA Jabs to ‘Turbo Cancer’
Several leading scientists have questioned the safety of the mRNA technology, including its inventor, Dr. Robert Malone, who believes the COVID-19 jabs are behind a surge in so-called “turbo cancers.”
Thousands more people are expected to be recruited to take part in mRNA trials, with NHS England saying the scheme will work with a range of pharmaceutical companies and could expand to include patients with other types of cancer.
Partnership With Genomics England
The latest trial is part of NHS England’s Cancer Vaccine Launch Pad, which is working in partnership with Genomics England to fast-track patients to receive the cancer jabs.
Patients wishing to take part will have a blood test and tissue sample taken. If considered eligible, they will be referred to their nearest NHS hospital involved in the scheme.
Thirty hospitals in England are currently signed up to the initiative, with more expected to join in the coming months.

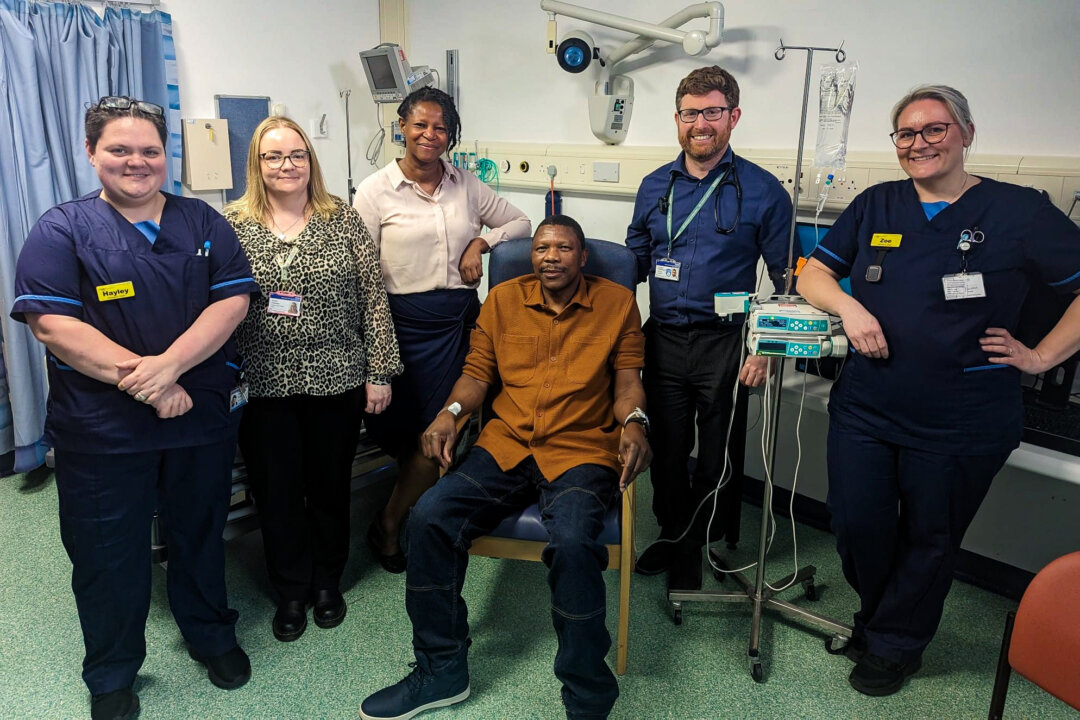
The first participant in the BioNTech-sponsored trial was lecturer Elliot Pfebve, 55, who had no symptoms but was diagnosed with cancer after a routine health check with his GP.
After having a 30-centimetre tumour removed from his large intestine, he was referred to the Queen Elizabeth Hospital Birmingham for chemotherapy and to take part in the clinical trial.
Mr. Pfebve, a father of four, said in a statement: “Through the potential of this trial, if it is successful, it may help thousands, if not millions, of people, so they can have hope and may not experience all I have gone through. I hope this will help other people.”
‘More Data Is Needed’
Dr. Victoria Kunene, a consultant clinical oncologist at Queen Elizabeth Hospital Birmingham and principal investigator for the trial, said in a statement: “The investigational cancer vaccines are based on mRNA and are created by analysing a patient’s tumour to identify mutations specific to their own cancer.
“Using this information, we can create an individualised investigational cancer vaccine, but it is too early yet to say if these will be successful, though we are extremely hopeful.
“Based on the limited data we currently have of the in-body response to the vaccine, this could prove to be a significant and positive development for patients, but more data is yet needed and we continue to recruit suitable patients to the trial to establish this further.”
NHS England Chief Executive Amanda Pritchard hailed watching Mr. Pfebve receive the vaccine as “a landmark moment for patients and the health service as we seek to develop better and more effective ways to stop this disease.”
She claimed that the NHS is in “a unique position” to deliver “this kind of world-leading research at size and scale, and as more of these trials get up and running at hospitals across the country, our national match-making service will ensure as many eligible patients as possible get the opportunity to access them.”
Trials have so far enrolled dozens of people, NHS England said, with the majority expected to take part from 2026 onwards.
Details of the trial were revealed ahead of the world’s largest cancer conference, the annual meeting of the American Society of Clinical Oncology, with tens of thousands of researchers and scientists set to convene in Chicago this weekend.