Lp(a) and SARS‐CoV‐2: A conspiracy of two mysteries
Since the beginning of the COVID-19 pandemic, more than 130 million people around the world have been infected with SARS-CoV-2. Initially, the disease outbreak presented many puzzling characteristics but during a very brief time, less than 1.5 years, our understanding of the virus, disease pathophysiology, roles of underlying risk factors and conditions impacting the clinical severity as well as avenues for treatment, prevention, and management of the disease have improved significantly [1]. It soon became clear that people living with chronic health conditions, such as diabetes mellitus, ischemic heart disease, cancer, hypertension, HIV infection, and respiratory diseases, have a greater risk of a worse outcome when infected with SARS-CoV-2. A particular prominent characteristic has been an increased incidence of thromboembolic events [2] and in addition, the occurrence of long-term complications that remain a challenge with many unanswered questions.
While many factors may eventually be identified that act synergistically with COVID-19 and accelerate underlying conditions, some of the features of the infection had similarities with the effects of lipoprotein(a) [Lp(a)], in particular the increase in cardiovascular and thromboembolic complications [3]. The basis for this synergy was the potential of Lp(a) to inhibit endogenous fibrinolysis through its apolipoprotein(a) [apo(a)] component and also to promote proinflammatory effects through its carrier role of proinflammatory and proatherogenic oxidized phospholipids (OxPL) [4]. This prompted a suggestion that a role for Lp(a) in this particular characteristic of the COVID-19 infection could be tested [5]. Furthermore, the LPA gene contains a response element to interleukin-6, an acute-phase cytokine, and the COVID-19-induced cytokine storm could potentially result in an acute phase-induced increase in Lp(a) levels. Thus, it might therefore be expected that people with elevated baseline Lp(a) acquiring a SARS-CoV-2 infection may be at a higher risk of developing thromboembolic events. In addition, an elevated Lp(a) level is associated with atherosclerosis, and further cardiovascular stress through a SARS-CoV-2 infection might result in destabilization of preexisting atherosclerotic plaques, promoting accelerated ischemic heart disease and the risk for myocardial infarction.
The current study by Maio et al., published in this issue [6], indeed tested such a potential synergy. The authors took advantage of one of the major advances during recent years, the publicly available datasets of large, population-based cohorts. Using data from the UK Biobank, the authors compared baseline Lp(a) levels in SARS-CoV-2 positive patients with those of COVID-19-negative or untested subjects and investigated the association of Lp(a) with cardiovascular or thromboembolic outcomes. While Lp(a) concentrations were similar between SARS-CoV-2 positive patients and negative or untested subjects, the risk for ischemic heart disease increased with elevated Lp(a) levels in both groups. Notably, this association was more apparent and steeper among patients with a SARS-CoV-2 infection, indicative of a modification of the association by the infection. Interestingly, although the frequency of thromboembolic events was five times higher among SARS-CoV-2 positive patients, no association was found between thromboembolic events and Lp(a) levels. The modulation of the relationship of Lp(a) with atherosclerotic or thromboembolic events in SARS-CoV-2-infected patients is illustrated in Fig. 1. The lack of any thromboembolic risk modification by Lp(a) is notable and does not provide support for a clinical role of Lp(a) for thromboembolic events. This potential effect of Lp(a), although a tempting hypothesis based on the molecular similarities with plasminogen, has been hard to pin down, although model animal or in vitro studies provide some support [3]. Clinical studies have been inconclusive and Mendelian randomization approaches have generally not provided support [7, 8].
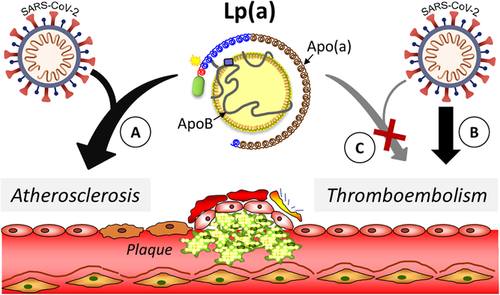
Modulation of the association of elevated Lp(a) concentrations with atherosclerotic but not with thromboembolic events by the SARS-CoV-2 infection. Lp(a) promotes cardiovascular risk through atherosclerotic and thromboembolic mechanisms. The SARS-CoV-2 infection enhanced the associations of elevated Lp(a) concentrations with atherosclerotic events such as ischemic heart disease, as evidenced by an apparent and steeper association (a). In contrast, while the SARS-CoV-2 infection increased the risks of the thromboembolic event by several fold in infected patients (b), these increased thromboembolic events were not associated with elevated Lp(a) concentrations (c).
The lack of a detectable difference in Lp(a) levels between COVID-19-positive and negative or untested subjects is also notable given the pro-inflammatory nature of COVID-19 and previous reports of higher Lp(a) levels in proinflammatory settings [9]. Some aspects to consider are the relatively short virus exposure time for the COVID-19-infected patients and a possible effect under more chronic exposure conditions cannot be excluded at present. Studies focussed on long-term sequelae resulting from a COVID-19 infection might provide more information. In contrast, the increasingly accepted role of Lp(a) as a prominent risk factor for atherosclerotic cardiovascular disease [10] is further strengthened by these studies. The mechanism underlying the results by Maio et al. remains to be elucidated but it is tempting to suggest that preconditioning of the vascular system by a lifelong exposure to Lp(a) may have prepared the ground for an additional, COVID-19-related insult. In light of this significant association in the absence of differences in Lp(a) concentrations between the infected group and the other groups, it would be interesting to study whether a SARS-CoV-2 infection might impact Lp(a) molecular properties, including its proinflammatory and proatherogenic OxPL content and/or endogenous enzymes that play important roles in homeostasis. Indeed, as Lp(a)-bound OxPL are hydrolyzed by lipoprotein-associated phospholipase A2, any impact on this pathway could potentially influence the biological activities of oxidized Lp(a) in the arterial wall, promoting atherogenesis and vascular damage. Although it is premature at this stage to draw any firm conclusions, further studies focussing on this issue would be of interest. Thus, while effects of Lp(a) in healthy settings (e.g., no infection, absent or low inflammation, and low oxidative stress) may be protective by collecting OxPL from various sources, leading to its degradation and removal, the effects under high-risk conditions (e.g., SARS-CoV-2 infection) might be opposite with increases in OxPL and lipoprotein-associated phospholipase A2 activity, contributing to an enhanced atherogenicity.
As the population tested was primarily Caucasian, it remains to be seen whether these findings will be generalizable to other population groups such as Hispanics or Blacks, two groups severely affected by SARS-CoV-2 infections and related complications. Notably, the latter group has consistently been found to have elevated Lp(a) levels [11]. Other limitations of the study were the inclusion of untested subjects, as many COVID-19-positive subjects were without symptoms and unaware of their exposure before testing results. The authors pointed out that results were obtained early in the epidemic arguing against this possibility but further studies are needed to confirm the results. Further, the analysis of Lp(a) was limited to plasma concentrations and no apo(a) isoform data were included. Overall, however, the study elegantly illustrates opportunities to utilize existing data sets and seize unexpected opportunities to enhance our insights into already existing risk mechanisms as well as to increase our understanding of the broader health impact of a COVID-19 infection. As we struggle to get a better understanding of long-term, downstream COVID-19 complications, studies like these are likely to shed more light.
Conflict of interest
The authors declare to have no conflict of interest.
Acknowledgments
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.