Pfizer and BioNTech ask FDA to authorize updated COVID booster for children
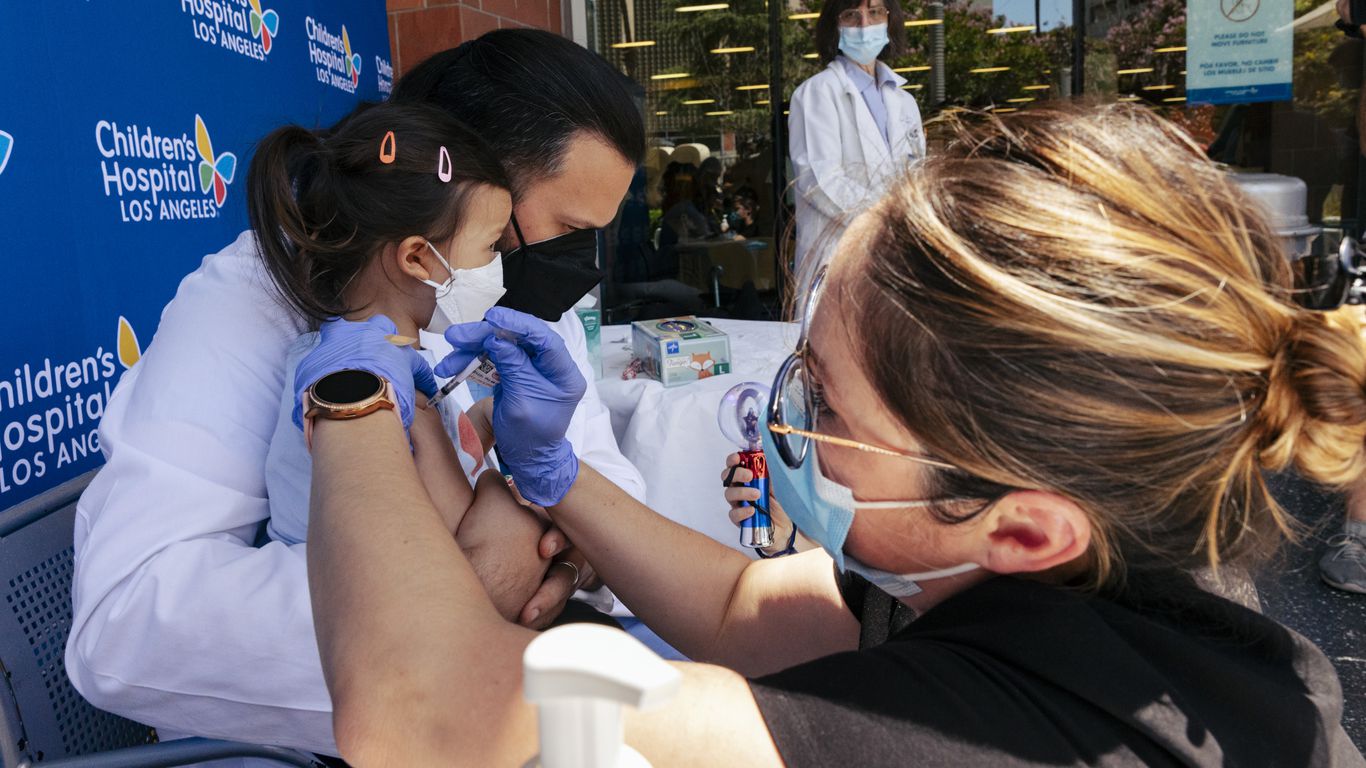
Pfizer and BioNTech announced Monday that they are seeking emergency use authorization from the FDA for its Omicron-specific COVID-19 booster for children ages 5-11.
The big picture: The companies’ submission comes after Moderna on Friday requested emergency use authorization for its Omicron-specific COVID booster shots for children 6-17 years old.
Driving the news: Pfizer and BioNTech also said Monday that they have started a study of different dosing regimens of the updated boosters in children 6 months to 11 years of age.
- The request is “supported by safety and immunogenicity data,” the companies said in a news release.
State of play: Vaccine uptake among older groups for the updated booster, called bivalent, has been slow. Approximately 4.4 million people have received updated boosters so far, which amounts to less than 2% of those eligible, per the CDC.
- However, that’s likely an undercount due to reporting lags in the states.
- Anybody over 12 was eligible to receive the Pfizer-BioNTech bivalent booster beginning in August.
Go deeper… Experts alarmed by COVID vaccination rates among America’s youngest kids