MHRA approves Bill Gates’ untried and untested South Korean covid vaccine
SKYCovion covid vaccine has just got regulatory approval from the UK Medicines and Healthcare products Regulatory Agency (“MHRA”). SKYCovion was developed in South Korea with significant funding from Bill Gates. It combines a part of the SARS-CoV-2 virus spike protein with an “adjuvant.” The self-assembled nanoparticle vaccine is adjuvanted with GSK’s AS03 adjuvant technology.
This vaccine appears to be targeted at underdeveloped countries. The MHRA states that: the duration of protection is unknown and no protection is anticipated after the first dose. Additionally, the safety and efficacy of the vaccine for immunocompromised people have not been assessed, there is no experience with the vaccine in pregnant women and it is unknown whether the vaccine is excreted in breastmilk.
So far, and unsurprisingly, SKYCovion has not been approved by any other Western country. So, Professor Norman Fenton is submitting a Freedom of Information request to MHRA Chief Executive June Raine to ask a few questions.
Related: WHO, Bill Gates and Wellcome Trust’s Global Vaccine Fund Lacks Transparency and Accountability
Let’s not lose touch…Your Government and Big Tech are actively trying to censor the information reported by The Exposé to serve their own needs. Subscribe now to make sure you receive the latest uncensored news in your inbox…
The New SKYCovion Vaccine: More Questions for MHRA to Answer
By Professor Norman Fenton
This is quite an alarming developing story that not enough people are paying attention to. With thanks to Debi Evans for this information.
In the segment starting at 47 mins on UK Column News 31 May 2023, Debi Evans discusses the new SKYCovion covid vaccine that has just got regulatory approval from the MHRA “after meeting the MHRA’s required safety, quality and effectiveness standards”. This is a so-called “preventative” vaccine that was made in South Korea with the help of Bill Gates.
Unlike the mRNA vaccines, this is a nanoparticle vaccine that is stored at 2-8C so no freezing or special equipment is needed. It is also cheap and easy to scale up:
As the promotional video suggests, this vaccine appears to be a primary course for those who have not had access to any vaccines and is targeted at underdeveloped countries. It is not designed for a highly vaccinated country like the UK.
The patient information leaflet, which lists various side effects, states that two doses are required (the second one 28 days after the first) and that there has to be a 15-minute observation period afterwards.
Particularly alarming points from the MHRA guidance document (in addition to the known side effects) are:
Duration of protection
The duration of protection afforded by the vaccine is unknown as it is still being determined by ongoing clinical trials.
Immunocompromised individuals
The efficacy, safety and immunogenicity of the vaccine have not been assessed in immunocompromised individuals, including those receiving immunosuppressant therapy.
Limitations of vaccine effectiveness
Based on immunogenicity data in SARS-CoV-2 naïve subjects, no protection is anticipated after the first vaccine dose and individuals may not be fully protected until 14 days after their second dose. As with all other vaccines, SKYCovion may not protect all vaccine recipients. Efficacy was not evaluated as part of the clinical trial programme.
Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed.
Concomitant administration with other vaccines has not been studied.
Pregnancy
There is no experience with the use of SKYCovion in pregnant women from clinical trials.
Breastfeeding
It is unknown whether SKYCovion is excreted in human milk.
So, it would seem nothing is known about its effectiveness (other than that, until at least 14 days after the second dose there is zero efficacy!) and almost nothing is known about its safety (and certainly there have been no carcinogenicity or genotoxicity studies). It has not been through any interchangeability studies with other manufacturers. It clearly should not be given to women who are pregnant or breastfeeding.
If that isn’t worrying enough, look at the qualitative and quantitative composition of the vaccine as stated in the guidance document from MHRA.
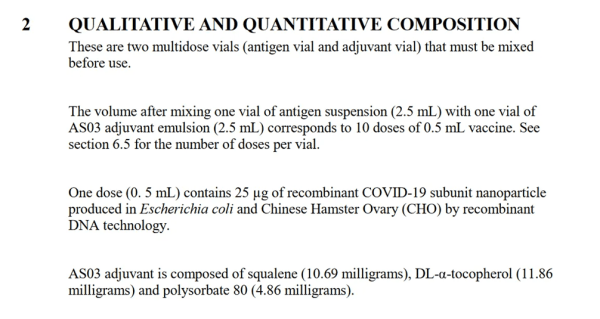
Note that there are two multi-dose vials that must be mixed and diluted before delivery. It is highly unlikely that patients will be given consistent and accurate doses. Also, note the adjutant contains squalene.
The vaccine, unsurprisingly, has not been approved by any other Western country, although the European Commission is now considering it. Yet the MHRA have licensed it under a conditional authorisation and for over 18s.
And it seems that, contrary to the suggestions otherwise, this vaccine is being offered to people who have already had multiple covid vaccines. How do we know this? Debi’s mother received a text message inviting her for a booster and it said: “We cannot tell you which vaccine you will receive, but it may contain squalene.” As far as we are aware none of the others contains squalene.
So why is the MHRA going against the manufacturer’s guidance and using a product that appears to have been designed for developing countries, with no known efficacy or safety?
Of course, as we made clear in this video, the MHRA under the leadership of June Raine has shifted from being a “regulator” to an “enabler.”
June Rain’s latest speech contains much of the same nonsense, but gives some indication as to how a vaccine like SKYCovion managed to get MHRA approval:
(More on the Changing World of the MHRA can be found in this lecture.)
We will be raising the above issues with June Raine and asking the following questions about the SKYCovion vaccine through a Freedom of Information Request:
1. You state that this vaccine was approved “after meeting the MHRA’s required safety, quality and effectiveness standards.” Yet your own guidance document makes clear that there is no efficacy data and minimal safety data for this vaccine. So, what exactly are the required safety, quality and effectiveness standard?
2. As the vaccine is primarily intended for people previously unvaccinated why did you authorise its use for a country like the UK that is highly vaccinated?
3. Are you dispensing the vaccine to already vaccinated people in the UK? If so, what studies have been performed to show that it is safe and effective to be used in people previously vaccinated with AstraZeneca, Pfizer, Moderna and other vaccine combinations?
4. As the vaccine is licensed to over 18s, and appears to be not recommended to women who are pregnant or breastfeeding, what information do you have about the effect of the vaccine on women who become pregnant sometime after vaccination?
5. What information do you have about the impact on the sperm count of men who receive this vaccine?
6. What studies have been performed on the sensitivity to safety and efficacy of the different dose accuracies that will inevitably result from the complex mixing process?
7. Did the change of role of the MHRA from regulator to enabler impact your decision to authorise this vaccine?
About the Author
Norman Fenton is a Professor Emeritus of Risk Information Management at the Queen Mary University of London. He is also a Director of Agena, a company that specialises in risk management for critical systems. He is a mathematician by training whose current focus is on critical decision-making and, in particular, on quantifying uncertainty using causal, probabilistic models that combine data and knowledge (Bayesian networks). The approach can be summarized as “smart data rather than big data.”
You can follow him on Twitter HERE. He also publishes articles on a Substack page titled ‘Where are the Numbers?’ which you can subscribe to and follow HERE.



