RFK Jr. resurrects an old antivax half-truth about “saline placebos” in randomized controlled trials of vaccines
This week, I could not avoid thinking yet again how much it is the case with antivaccine messaging since the COVID-19 pandemic hit that “everything old is new again.” Unfortunately, this would appear to be more the case than ever since longtime antivaccine activist Robert F. Kennedy, Jr. announced that he was challenging President Joseph Biden for the 2024 Democratic nomination for President. Unfortunately, his move has led to more publicity than ever before for his antivaccine beliefs and conspiracy theories. (Just Google his name if you don’t believe me.) While it is true that some media outlets are appropriately reporting his antivaccine conspiracy theories (and many other conspiracy theories and lies) for what they are, totally bonkers, much of the media is also being unduly respectful of his tinfoil hat worthy ideas—even stories that report on his conspiracy theories also can’t resist the Kennedy allure enough not to include photos of him as a child with his father and/or President John F. Kennedy and portray the ostracism that he experiences because of his antivaccine views as him “sacrificing” to speak up for what he believes—while RFK Jr. has also managed to attract what Judd Legum appropriately characterizes as a “pernicious obsession” by elites. Despite his previous reputation as a lefty environmentalist RFK Jr. also epitomizes the far rightward shift of the political center of the antivaccine movement over the last decade or so, having embraced issues besides resistance to vaccine mandates that traditionally championed by the right, such as cryptocurrency, antimask and anti-“lockdown” views, and even free market fundamentalism. The interesting thing is that initially RFK Jr. assiduously avoided the issue of his long history of antivaccine activism but of late seems to have not just acknowledged it but made it a centerpiece of his campaign, leading to vigorous discussions about how the media has failed and can “ethically” and responsibly cover his campaign.
Unfortunately, RFK Jr. is clearly exulting in the attention, which is more publicity and coverage than he has ever enjoyed in his entire life, making me think that when he inevitably loses the Democratic nomination he’ll mount a third-party candidacy to keep the attention and cash rolling in. Worse, he’s taking full advantage of it to resurrect old antivaccine misinformation. Indeed, he’s partying like it’s 2005 (apologies to Prince) by recycling old debunked claims about mercury in vaccines causing autism, vaccines in general causing autism, and all manner of other antivaccine claims. During his appearance on Joe Rogan’s podcast last month, he made the claim that almost none of the vaccines currently on the childhood vaccine schedule has been tested in randomized controlled trials (RCTs) against a saline placebo. Clinical trialists, even ones who know very little of the specifics of the clinical trials used to approve vaccines, will likely immediately recognize the simplistic assumption at the heart of what I like to call the “no true saline placebo” trope: That the only appropriate placebo in a randomized controlled trial (RCT) of an injectable medication or vaccine is saline and that anything else is suspect. Learning a bit about the specific subject, they would soon also realize that a very important bit of context is missing from this narrative, specifically the issue of clinical equipoise and the ethics of clinical trials.
As I will explain, the “no saline placebo control” claim is a classic antivax half-truth that sounds ominous only if you do not understand the ethical requirements that must be fulfilled in order to justify doing any RCT and what constitutes a valid placebo or control group in such trials. (Indeed, Dr. Paul Offit’s term for this trope by antivaxxers, the “casual cruelty of placebo-controlled trials,” is quite apt.) The “no saline placebo control” gambit was used by antivaccine activist Del Bigtree in 2019 to call into doubt the licensing of some MMR vaccines and by Peter Götzsche to cast doubt on the safety of Gardasil. (Never mind that the existence of long term studies like this one that did compare HPV vaccines to saline placebo controls rather undermines this particular antivaccine talking point.) Readers of my not-so-super-secret other blog will recognized that I have discussed this issue recently, but I consider this issue so important that it needs to be discussed on SBM. Also I now realize that I didn’t expend enough verbiage discussing one aspect of this trope, namely the false claim that placebos and comparators other than saline are used in an nefarious plot to “hide” adverse reactions in the vaccine group compared to control. Consider this a fairly heavily revised and expanded version of that post from last week.
First, let’s recap what an RCT is, how RCTs are carried out, and where they fit in the scheme of the evidence required for FDA approval of a drug. You can skip the next section if you are familiar with RCTs and drug regulation.
What are randomized controlled clinical trials (RCTs)?
Randomized controlled clinical trials are just that: clinical trials in which subjects are randomized to receive either the intervention or a control (such as a saline placebo) and then followed to determine which group has better outcomes, which are prespecified in the clinical trial protocol. The reason for randomization is to ensure that the two groups being compared resemble each other as closely as possible in characteristics relevant to the outcomes being tested. For example, if you’re testing a drug to treat hypertension, you would want the groups to be matched as closely as possible for, among other characteristics, age, race, sex, severity of hypertension, and relevant risk factors for poor outcomes. Ideally, these RCTs are then double-blinded, so that neither the subjects nor the doctors or medical personnel administering the drugs and assessing outcomes know which group any given subject is in. Double blinding is especially important in clinical trials with more subjective outcomes such as pain, for which placebo effects can be strong, but it’s also important even in trials with “hard” outcomes like tumor progression because it could affect how clinicians interpret tests and radiology studies if they know which group a given patient is in. Moreover, such clinical trials have strict inclusion and exclusion criteria, which ensure that those being treated actually have the disease, do not belong to a group that might be harmed by the drug, and are subjects who are likely to benefit if the drug does have efficacy; i.e., does work.
RCTs occur relatively late in the process of drug development, particularly large phase 3 RCTs. In general, during drug development promising compounds are first tested in what are called phase 1 trials, which involve giving the drug to small numbers of patients at different doses to find (1) obvious severe toxicities and (2) an optimal dose to test for RCTs, which is why such trials are often also serve as dose-finding studies to determine the drug dose to use in the early RCTs done in phase 2 and the large RCTs done in phase 3. Note that phase 1 trials do not look for efficacy; that is not their purpose. If they find evidence of efficacy, that’s all great and promising, but it’s not expected. The number of subjects is too low, and they are generally not randomized. Phase 2 clinical trials are generally smaller RCTs that serve as the first real test of whether the drug works. If the drug shows promise in phase 2, then large phase 3 RCTs are carried out. In the case of vaccines, these RCTs often involve tens of thousands of subjects.
I like to refer to this graphic from this article to sum it up:
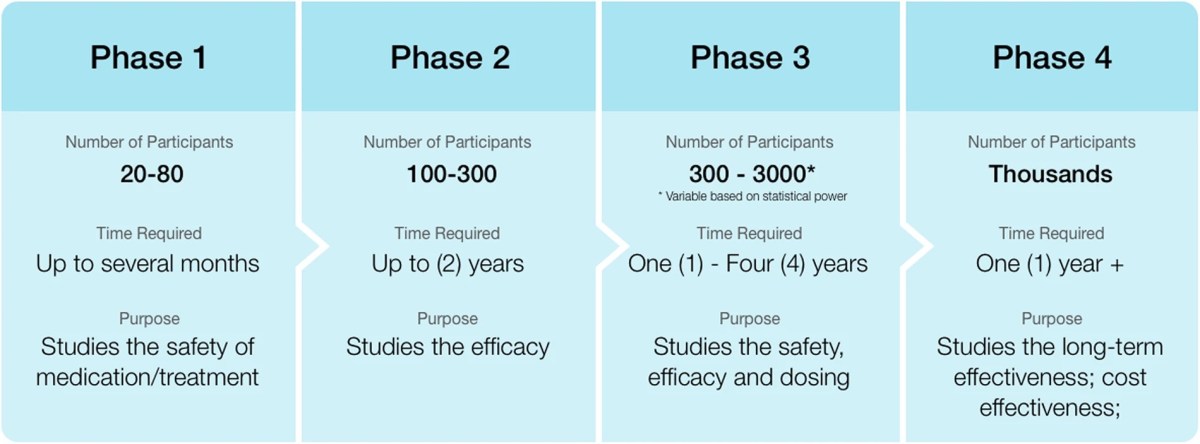
This is the traditional four phase clinical trial paradigm. (Phase 4 studies are postmarketing studies that look for safety signals missed in the studies used for FDA approval.)
The above is, of course, the simple, “classic” paradigm. Over the years, scientists have developed a number of variations, such as combining phase 1 and 2 trials, adaptive trial designs, and other designs that can test multiple outcomes. Also note that the reason for phase 4 postmarking studies is that even large phase 3 RCTs can miss rare adverse events and efficacy is also often lower than in RCTs once a treatment is released “out into the wild,” so to speak, and inevitably given to patients who might not have fit the strict inclusion and exclusion criteria of the RCTs used to approve it.
Let’s take a look at a practical hypothetical example. Let’s say I’ve developed a new drug that preclinical evidence suggests to me will be an effective treatment for a given condition, say X Disease. To win FDA approval for that drug to treat the specific indication of X Disease, I would propose an RCT in which I recruit patients with X Disease, randomize them into a group receiving the new drug or a control group receiving a placebo. Then I would follow the two groups and see which one does better with respect to X Disease. What percentage, for instance, of the treated group gets better compared to what percentage of the control group? What percentage of the treated group dies of X Disease compared to the percentage in the control group that dies? What percentage of subjects in the treatment group suffers known complications of X Disease compared to controls? As you might imagine, it gets a lot more complicated than that fairly fast, and I will discuss those complications in a moment because widespread ignorance of them are what the “no true saline placebo” trope depends upon. Basically, what I have presented is an idealized version of what an RCT is and how it works, because that is what most lay people understand about RCTs; that is, if they even know what an RCT is at all, just as their understanding of what constitutes an acceptable placebo to use for a placebo control is the simplest “ideal” placebo; i.e., completely “inert,” like saline.
Also contributing to the effectiveness of the “no saline placebo” trope is that many educated lay people are also at least vaguely aware that in the evidence-based medicine (EBM) paradigm, RCTs are near the top of the pyramid for strength of evidence. Systematic reviews and meta-analyses of RCTs are higher, but as individual studies rather than existing studies lumped together RCTs are at the top.) So when they hear antivaxxers claim and show that there are vaccines on the childhood vaccine schedule that have never been tested against a “saline placebo” in an RCT, it sounds fishy to them. Why weren’t these vaccines tested against a saline placebo? What were those evil pharmaceutical companies doing? Why did the FDA let them get away with it? It’s all understandable that one might become suspicious if one doesn’t know much about the other considerations that must go into an RCT.
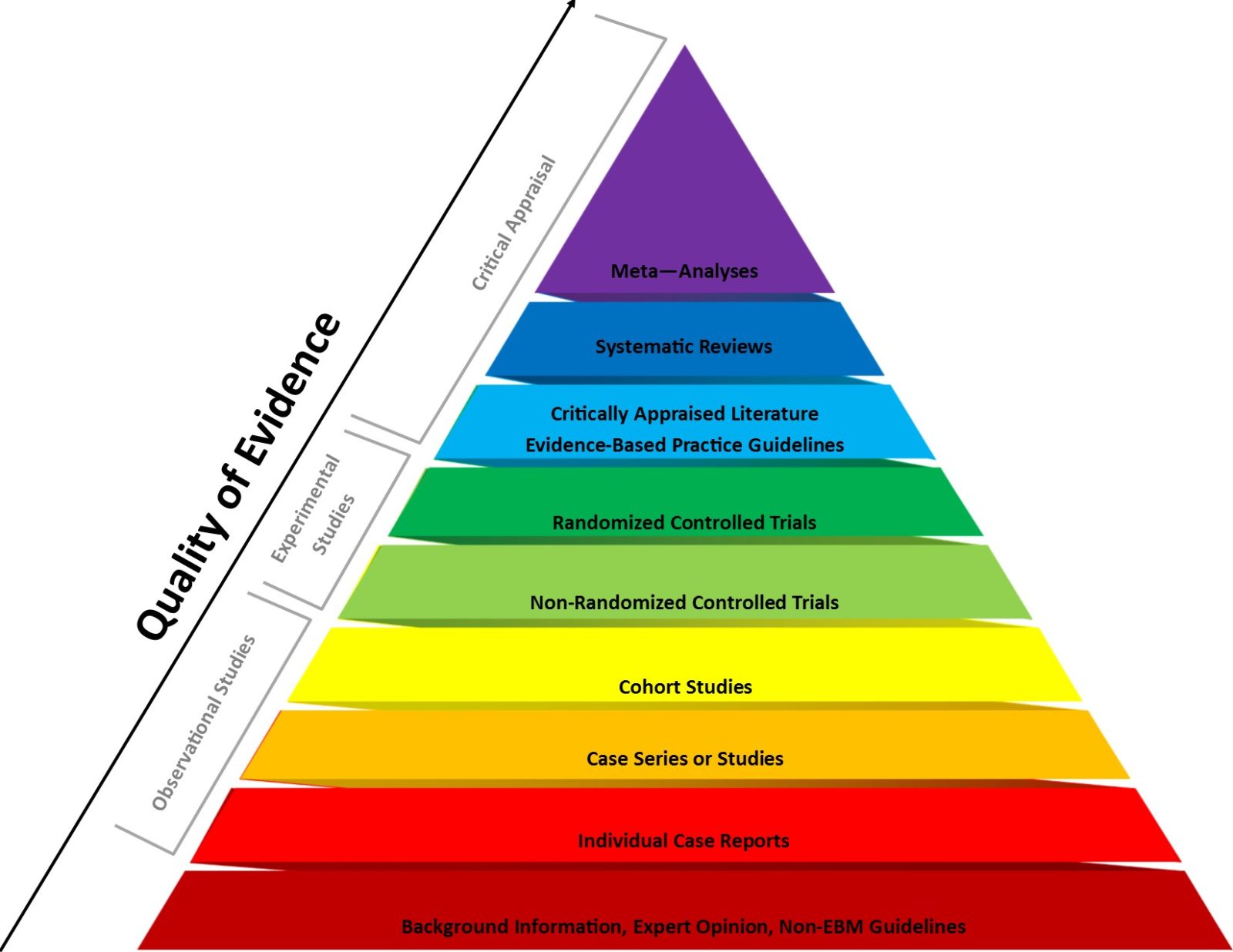
EBM pyramid of evidence. RCTs are not quite at the top, but note that systematic reviews and meta-analyses that lump together RCTs are at the top
Let’s look at one article—on Substack, of course—that is popping up all over the place, in particular the chart included with the article. It’s an article by Aaron Siri, the attorney for ICAN, Del Bigtree’s antivaccine organization, and it is directly of a piece with ICAN’s prepandemic attempts to spread fear, uncertainty, and doubt about MMR vaccines.
Robert F. Kennedy Jr. and the “no saline placebo” gambit
What brought this to my attention was the response to pediatrician, vaccine scientist, and advocate Dr. Paul Offit’s Substack post outlining the many lies of Robert F. Kennedy, Jr., who has of late taken up the “no true saline placebo” gambit as supposed evidence that the vaccines in the childhood schedule were somehow not thoroughly tested and therefore have unproven safety and efficacy:
This is not quite the “gotcha” that Mr. Siri thinks that it is, although Dr. Offit was a bit sloppy in his post refuting RFK Jr., a mistake he made up for with his post on the casual cruelty of calling for saline placebo RCTs for everything. To me this example is a good cautionary tale about how even someone as knowledgeable about vaccine development and approval as Dr. Offit can be tripped up by being insufficiently careful in his language. But who is Aaron Siri? First of all, he is the author of a deceptive Substack post making the rounds entitled Clinical Trial to License RotaTeq, Like Almost All Childhood Vaccines, Did Not Use a Placebo Control, in which he concludes that “those attacking RFK Jr. are wrong.” More importantly, he is an antivax lawyer who describes himself as “Managing Partner of Siri & Glimstad, has extensive complex civil litigation experience, including civil rights involving mandated medicine, class actions, and high stakes disputes.” He is, however, ICAN’s lawyer, which should tell you all you need to know about his proclivities and the deceptive arguments he uses.
The main part of his Substack article that’s gone viral is this chart:
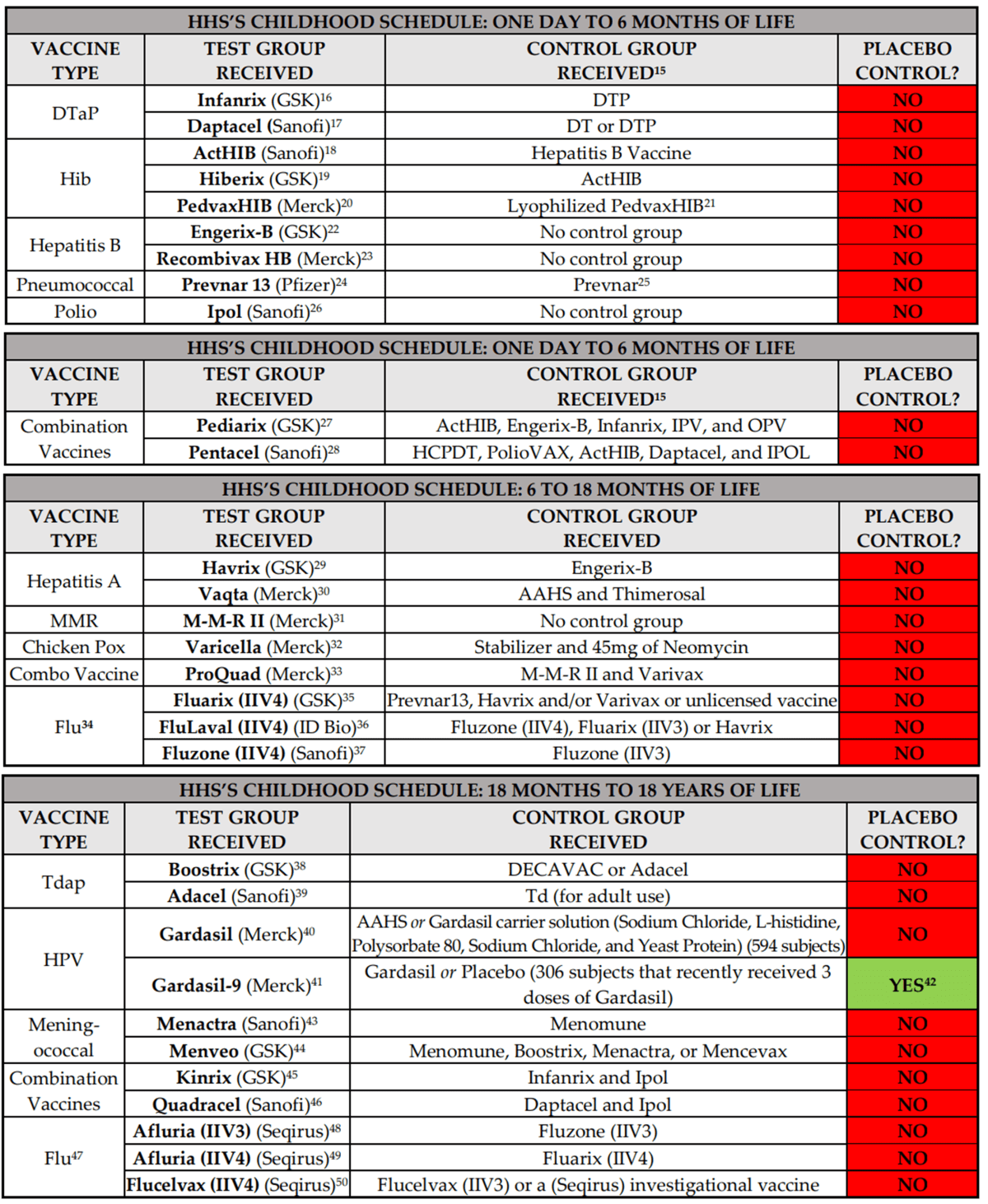
This is what I like to call lying with mostly accurate information presented without proper scientific context.
Siri, being a lawyer, makes a deceptive lawyerly opening statement:
Robert F. Kennedy, Jr. is on record stating that almost all childhood vaccines were licensed based on clinical trials that did not include a placebo control. He is correct.
Nonetheless, numerous news outlets, such as Stat News in its article titled “Correcting Robert F. Kennedy Jr.’s vaccine ‘facts’”, are stating Mr. Kennedy is wrong because they claim the clinical trial relied upon to license the rotavirus vaccine, RotaTeq, did include a placebo control. They are wrong.
A placebo is defined by the CDC as a “substance or treatment that has no effect on living beings.” This means a saline injection or water drops in mouth.
Again, the CDC appears to have fallen into the trap of using way too simplistic a definition of placebo. “No effect on living beings” is truly a cringeworthy definition. There are, in fact, a number of considerations that go into choosing a placebo, and, in fact, placebos are not always chosen because they are “completely inert.” For example, sometimes you don’t want a placebo to be “completely inert” because then it will be too easy for clinical trial subjects and the doctors and nurses administering the experimental therapeutic to figure out whether any given subject has been randomized to the control or experimental group! Moreover, not even saline is “completely inert”! If, for instance, you give enough saline you can cause fluid overload, hypernatremia (too much sodium) and a number of other toxicities. I could go on and note that not even the classic example of a sugar pill used as a placebo in RCTs of orally administered drugs is “completely inert.” I will instead argue that a much better way of looking at a placebo control is that it should be “inert” with respect to the outcomes being studied in the RCT.
Why do we use placebos in the first place? I like this description, provided in these clinical trial guidelines Tweeted by Ed Nirenberg:
In a placebo-controlled trial, subjects are randomly assigned to a test treatment or to an identical-appearing treatment that does not contain the test drug. The treatments may be titrated to effect or tolerance, or may be given at one or more fixed doses. Such trials are almost always double-blind. The name of the control suggests that its purpose is to control for “placebo” effect (improvement in a subject resulting from thinking that he or she is taking a drug), but that is not its only or major benefit. Rather, the placebo control design, by allowing blinding and randomization and including a group that receives an inert treatment, controls for all potential influences on the actual or apparent course of the disease other than those arising from the pharmacologic action of the test drug. These influences include spontaneous change (natural history of the disease and regression to the mean), subject or investigator expectations, the effect of being in a trial, use of other therapy, and subjective elements of diagnosis or assessment. Placebo-controlled trials seek to show a difference between treatments when they are studying effectiveness, but may also seek to show lack of difference (of specified size) in evaluating a safety measurement. In that case, the question of whether the trial could have shown such a difference if there had been one is critical (see section 1.5).
Again, “inert” does not have to mean “completely inert” or “no effect on living beings.” In fairness, though, the FDA definition for the public (an “inactive pill” that is “sometimes called a “‘sugar pill’”) is not much better, as it is also wildly simplistic too. What really matters for the purpose of FDA approval of a vaccine or any drug are FDA regulations for what constitutes a well-controlled clinical trial. I’m going to quote in full the relevant section, which not coincidentally is similar to what clinical trialists generally consider well-controlled clinical trials:
(2) The study uses a design that permits a valid comparison with a control to provide a quantitative assessment of drug effect. The protocol for the study and report of results should describe the study design precisely; for example, duration of treatment periods, whether treatments are parallel, sequential, or crossover, and whether the sample size is predetermined or based upon some interim analysis. Generally, the following types of control are recognized:
(i) Placebo concurrent control. The test drug is compared with an inactive preparation designed to resemble the test drug as far as possible. A placebo-controlled study may include additional treatment groups, such as an active treatment control or a dose-comparison control, and usually includes randomization and blinding of patients or investigators, or both.
(ii) Dose-comparison concurrent control. At least two doses of the drug are compared. A dose-comparison study may include additional treatment groups, such as placebo control or active control. Dose-comparison trials usually include randomization and blinding of patients or investigators, or both.
(iii) No treatment concurrent control. Where objective measurements of effectiveness are available and placebo effect is negligible, the test drug is compared with no treatment. No treatment concurrent control trials usually include randomization.
(iv) Active treatment concurrent control. The test drug is compared with known effective therapy; for example, where the condition treated is such that administration of placebo or no treatment would be contrary to the interest of the patient. An active treatment study may include additional treatment groups, however, such as a placebo control or a dose-comparison control. Active treatment trials usually include randomization and blinding of patients or investigators, or both. If the intent of the trial is to show similarity of the test and control drugs, the report of the study should assess the ability of the study to have detected a difference between treatments. Similarity of test drug and active control can mean either that both drugs were effective or that neither was effective. The analysis of the study should explain why the drugs should be considered effective in the study, for example, by reference to results in previous placebo-controlled studies of the active control drug.
(v) Historical control. The results of treatment with the test drug are compared with experience historically derived from the adequately documented natural history of the disease or condition, or from the results of active treatment, in comparable patients or populations. Because historical control populations usually cannot be as well assessed with respect to pertinent variables as can concurrent control populations, historical control designs are usually reserved for special circumstances. Examples include studies of diseases with high and predictable mortality (for example, certain malignancies) and studies in which the effect of the drug is self-evident (general anesthetics, drug metabolism).
Note the part about the placebo being an “inactive preparation designed to resemble the test drug as far as possible”; that is not necessarily “completely inert.” My point in citing this is to emphasize that there are a number of scientifically acceptable control groups other than placebo and that for vaccine trials there are a number of potential control groups other than a saline placebo. I will elaborate in the next section.
Clinical equipoise: How does that work?
RFK Jr. and antivaxxers like Mr. Siri would have you believe that the only consideration that goes into clinical trial design is rigorous science, which always demands that one use a “completely inert” placebo that, in the case of injectable drugs like vaccines, must be saline. This is a risibly simplistic view of drug and vaccine development and clinical trials, chosen intentionally to be used as misinformation about vaccines and ignores a very important standard that all clinical trials, in particular RCTs, must meet: Clinical equipoise.
Let me start with an example to illustrate the concept. Let’s say we have a new chemotherapy drug for breast cancer that we want to test in a clinical trial. If you were to accept Mr. Siri’s intentionally simplistic view of RCTs, you would think that any RCT testing this new drug must be designed with a placebo control group that receives only saline placebo. You would be so very, very incredibly wrong. The reason is that such a trial would be totally unethical, for the simple reason that you would be leaving cancer patients in the control group untreated. You can’t ethically randomize subjects to a group that will deny them existing effective treatment and thus cause them harm.
This is why many cancer therapies are tested in so-called add-on RCTs, as described by the guidelines I cited above and that could be fit into the FDA regulation that allows an active treatment concurrent control:
The use of a placebo control group does not imply that the control group is untreated. In many placebo-controlled trials, the new treatment and placebo are each added to a common standard therapy (so called add-on studies, see section 2.1.5.2.1).
This design means that the placebo group still receives at least the existing standard-of-care treatment for their cancer and is not intentionally left untreated—or even just undertreated. However, we might have reason to believe that the new treatment is as good or better than existing standard-of-care treatment but that adding it to these drugs would increase toxicity beyond what is tolerable. In this case, the proper control group is not a saline placebo control that would leave the group untreated but to compare the new drug against standard-of-care:
In an active control (or positive control) trial, subjects are randomly assigned to the test treatment or to an active control treatment. Such trials are usually double-blind, but this is not always possible; many oncology trials, for example, are considered difficult or impossible to blind because of different regimens, different routes of administration (see section 1.3.2), and different toxicities. Active control trials can have two distinct objectives with respect to showing efficacy: (1) to show efficacy of the test treatment by showing it is as good as a known effective treatment or (2) to show efficacy by showing superiority of the test treatment to the active control. They may also be used with the primary objective of comparing the efficacy and/or safety of the two treatments (see section 1.4). Whether the purpose of the trial is to show efficacy of the new treatment or to compare two treatments, the question of whether the trial would be capable of distinguishing effective from less effective or ineffective treatments is critical (see section 1.5).
To expand upon this explanation, the design of these sorts of studies is either what we refer to as “non-inferiority,” in which the goal is to show that the new treatment is at least not inferior to—or, colloquially, is at least as good as—the existing standard of care. Given such a result, there might be reasons to favor the new treatment even if it is only “as good” as the older treatment if, for example, it’s cheaper, easier to dose, or has a more tolerable side effect profile. Of course, ideally, we would prefer to be able to show that the new drug is better than the existing standard-of-care, but this is often very difficult to do, which is why noninferiority trials are more common.
In any event, regardless of the scientific rigor of the trial, randomization requires clinical equipoise, the existence of genuine uncertainty based on existing science and evidence about which group, control or experimental, will do better. Clinical equipoise is not just a requirement, but absolute requirement for randomization of clinical trial subjects to be considered ethical. Note that clinical equipoise does not mean that there is no evidence that a treatment works or is better than existing treatments. After all, the reason we do clinical trials is to find drugs that work where there currently are none and to find drugs that work better than current therapy, and there has to be compelling preclinical evidence from laboratory and animal studies, as well as preliminary early stage clinical trials, to justify testing a drug or vaccine in an RCT. There also has to be genuine scientific uncertainty, even if, as is usually the case, investigators think that it is more likely than not that the experimental group will do better than the control group. Absent that legitimate uncertainty, an RCT to test an intervention is unethical, as is the case in randomizing a cancer patient to be untreated or a child to be left unprotected against measles.
That brings us to vaccines. The only time that a placebo-controlled RCT of a vaccine can be ethically justified is when there currently does not exist a safe and effective vaccine against the disease for which the experimental vaccine has been designed. Again, just as it is unethical to randomize a cancer patient to a placebo control group when there exists effective chemotherapy that is standard-of-care, so too is it unethical to randomize a subject to a saline placebo group in a vaccine RCT when there already exists a licensed vaccine that is considered standard-of-care for the same disease. It is unethical to leave a child unprotected against measles in order to test a new measles vaccine. My guess is that Mr. Siri probably knows this but leaves it out. Either that, or, like most antivaxxers, he does not accept that reasoning because he believes vaccines are more harmful than the diseases against which they protect.
Now look at Mr. Siri’s list above that’s been going around social media. Do you notice anything? Almost none of the vaccines on the list that weren’t licensed based on RCTs using saline controls are not first generation vaccines, which means that it would have been unethical to test them against a saline control. So they were tested against some other comparator, usually an earlier licensed version of a vaccine against the same disease, for example DTaP vaccines being tested against the older DTP vaccines, flu vaccines like Fluvaria being tested against older flu vaccines like Fluarix or Fluzone, or Hiberix being tested against older vaccines against Hib like ActHIB. The bottom line is that, if you trace back the history of the vaccines developed for a disease like, say, measles, you will eventually find the RCT testing the first effective vaccine against it and that vaccine will have had a placebo control. It might not have been saline (although in most cases decades ago it was), but it will have been a placebo that was “inert” with respect to preventing that disease. Also, clinical trial standards have evolved over the last 70 years. If a vaccine was approved 60+ years ago using methodology that today we might consider inadequate, that does not change the calculus when it comes to testing new vaccines against the same disease. Such vaccines can’t ethically be tested against saline placebo.
When I first decided to write this post, I initially thought that I’d have to look up each and every clinical trial on the table, but then I realized that that’s not necessary, at least not initially. All I really need to do is to show how intellectually dishonest this narrative by RFK Jr. and Aaron Siri is by pointing out that a lot of current vaccines were never tested against a saline placebo control and that’s OK. The reason it’s OK is because clinical trial ethics, specifically clinical equipoise, demanded it. It is unethical to use a saline placebo control for an RCT of any vaccine that is not a brand new vaccine against a disease for which there are currently no safe and effective vaccines. It might well be necessary to go through each example in a subsequent post, but for now I will simply point to one of my favorite resources, Vaxopedia, to emphasize that, in fact, there are lots of RCTs of vaccines, childhood and adult, that did use saline or other valid “inert” placebos. Dr. Vincent Iannelli asks: Why isn’t every vaccine on the immunization schedule or every combination of vaccines tested using a double-blind, placebo controlled study?
The question is answered by a WHO expert panel:
Placebo use in vaccine trials is clearly acceptable when (a) no efficacious and safe vaccine exists and (b) the vaccine under consideration is intended to benefit the population in which the vaccine is to be tested. In this situation, a placebo-controlled trial addresses the locally relevant question regarding the extent to which the new vaccine is better than nothing, and participants in the placebo arm of the trial are not deprived of the clinical benefits of an existing efficacious vaccine.
Placebo use in vaccine trials is clearly unacceptable when (a) a highly efficacious and safe vaccine exists and is currently accessible in the public health system of the country in which the trial is planned and (b) the risks to participants of delaying or foregoing the available vaccine cannot be adequately minimized or mitigated (e.g. by providing counselling and education on behavioural disease prevention strategies, or ensuring adequate treatment for the condition under study to prevent serious harm). In this situation, a placebo-controlled trial would not address a question that is relevant in the local context, namely how the new vaccine compares to the one that is currently in use, and participants would be exposed to unacceptable levels of risk from delaying or foregoing a safe and effective vaccine that is accessible through the public health system.
Antivaxxers like RFK Jr., Aaron Siri, and Del Bigtree take advantage of the public’s lack of understanding that ethical requirements to justify an RCT must reign supreme and that a lack of a saline control of a current non-first-generation vaccine does not mean that the vaccine is unsafe or that anything nefarious was done to win FDA approval. It is also an outright lie to claim that vaccines are not tested adequately for safety.
A scientifically valid placebo doesn’t have to be saline
But, wait! What about the choice of placebo? A frequent retort to the explanation that you can’t ethically do an RCT of a vaccine for all the reasons enumerated above is the false claim that not using a saline placebo is intended to “hide” adverse reactions because there will be adverse reactions to the comparator used in the control group of the RCT. Consider this trope a variant of the “toxins gambit,” that old antivax trope favored by Jenny McCarthy back in the day that portrays the ingredients of vaccines other than the antigen used to provoke the protective immune response to the pathogen as, at worse, horribly toxic and, at best, untested and therefore of unproven safety.
As I pointed out above, RFK Jr., Del Bigtree, and Aaron Siri would have you believe that saline is the only scientifically acceptable comparator for a control group in an RCT, but such is not the case and, as I’ve pointed out, earlier versions of many vaccines have been tested against saline placebos. But why do we use placebos in the first place? I like this description, provided in these clinical trial guidelines:
In a placebo-controlled trial, subjects are randomly assigned to a test treatment or to an identical-appearing treatment that does not contain the test drug. The treatments may be titrated to effect or tolerance, or may be given at one or more fixed doses. Such trials are almost always double-blind. The name of the control suggests that its purpose is to control for “placebo” effect (improvement in a subject resulting from thinking that he or she is taking a drug), but that is not its only or major benefit. Rather, the placebo control design, by allowing blinding and randomization and including a group that receives an inert treatment, controls for all potential influences on the actual or apparent course of the disease other than those arising from the pharmacologic action of the test drug. These influences include spontaneous change (natural history of the disease and regression to the mean), subject or investigator expectations, the effect of being in a trial, use of other therapy, and subjective elements of diagnosis or assessment. Placebo-controlled trials seek to show a difference between treatments when they are studying effectiveness, but may also seek to show lack of difference (of specified size) in evaluating a safety measurement. In that case, the question of whether the trial could have shown such a difference if there had been one is critical (see section 1.5).
Let me emphasize one more time that “inert” does not mean “completely inert” or “no effect on living beings,” as the CDC definition simplistically states. Let me describe one example. It is not uncommon for vaccine trials to use a placebo that contains every ingredient in the vaccine except the active ingredient, the antigen chosen to incite an immune response against the targeted pathogen. The placebo will thus contain buffer, saline, whatever adjuvant is used (if an adjuvant is used), and all the other ingredients in the vaccine. One advantage of this choice is that, unlike the case with a placebo injection that is unlikely to cause a reaction (e.g. saline), many subjects receiving the placebo will have a reaction at the injection site similar to that caused by the complete vaccine and will thus be less likely to guess correctly about whether they are in the placebo group or not. The second advantage of such a design is that it demonstrates specificity. If the trial is positive, then one can say confidently that the protective effect against disease was due to the antigen in the whole vaccine, not any nonspecific effects due to any or all of the rest of the ingredients. Antivaxxers hate this design because they believe incorrectly that it is meant to deceive, because to them, despite their impressive six decade record of safety, aluminum adjuvants (for example) are dangerous and part of what antivaxxers view as the toxic brew that causes autism, sudden infant death syndrome (SIDS), etc. Contrary to antivax claims, there is no good evidence linking aluminum adjuvants to autoimmune disease or allergies or that they are unsafe, a recent oft-cited (by antivaxxers) observational study suggesting a link to asthma notwithstanding.
If you want to see just how ridiculously far someone like Aaron Siri will go to cast doubt upon even the most unobjectionable placebo, let’s go back to his attack on Dr. Paul Offit and the vaccine that he co-invented, RotaTeq:
These same four ingredients are also contained in RotaTeq. The only difference between the vaccine and the control is that RotaTeq also included tissue culture medium and rotavirus reassortments. So, bottom line: the control used in the RotaTeq clinical trial was not a placebo since it included bioactive ingredients.
He then goes into incredible contortions to portray sodium phosphate as dangerous:
For example, here is what the NIH explains about sodium phosphate, one of the ingredients in the control:
Sodium Phosphate can cause serious kidney damage and possibly death. In some cases, this damage was permanent, and some people whose kidneys were damaged had to be treated with dialysis (treatment to remove waste from the blood when the kidneys are not working well). Some people developed kidney damage within a few days after their treatment, and others developed kidney damage up to several months after their treatment.
I apologize to any nephrologist out there who just spit up their drink onto their laptop after having read the above passage. Let’s just say that Mr. Siri left out a very important bit of context, the dose of sodium phosphate required to cause these harms. The amount of sodium phosphate used to buffer vaccines is minuscule compared to the huge amount of phosphate administered in a colonoscopy prep (which is the context of the document cited). I trust that readers will recognize that milligram quantities of sodium phosphate are orders of magnitude lower than the gram quantities used in colonoscopy preps (each tablet is 1.5 g). It’s also a nice bit of deceptive appeal to authority to represent this as being the NIH’s proclamation. Medline Plus is published by the National Library of Medicine, which is part of the NIH, making it technically the NIH, but to represent this document as some sort of definitive proclamation of the NIH is another example of deceit by leaving out context.
It’s basically the same gambit antivaxxers have long loved to invoke to make it sound as though formaldehyde in some vaccines is a horrific toxin, even though the amount in a childhood vaccine is minute:
Assuming an average weight of a 2-month-old of 5 kg and an average blood volume of 85 ml per kg, the total quantity of formaldehyde found in an infant’s circulation would be about 1.1 mg, a value about 1,500 times more than the amount an infant would be exposed to in any individual vaccine.
Siri pulls the same deceptive trick with polysorbate-80:
And as these studies and data sheet make clear, polysorbate-80 is far from an inert substance, is bioactive, and can have safety concerns, especially when given to infants.
Bottom line, Robert F. Kennedy, Jr.’s claim that virtually all childhood vaccines were licensed based on clinical trials that did not include a control group that received a placebo is correct. The undisputable evidence for this claim, all from FDA or pharma sources, is detailed on pages 3 to 7 of a response we sent to HHS on December 31, 2018.
One of the studies listed by Siri is an animal study from 1992 that gave rats huge doses of polysorbate-80. The Material Safety Data Sheet referenced actually notes that the compound is not “a dangerous substance or mixture according to the Globally Harmonized System (GHS)” and that the product “does not contain any hazardous materials with occupational exposure limits established by the region specific regulatory bodies.” One must also remember that safety sheets like this are intended for laboratory and manufacturing personnel who come into contact with large amounts of the compound, not with the milligram quantities in the RotaTeq placebo. The other reference is a review article that reviewed the scientific literature to determine safe doses of polysorbate-80 in children based on large exposures to the compound associated with intravenous drugs like the cardiac antiarrhythmic amiodarone, chemotherapeutics like docetaxel, and intravenous vitamin E. We are talking about large doses, too. For example:
The increase in hypersensitivity reactions to docetaxel is likely to have been caused by extremely high doses of PS80. Based on the FDA-approved dose of docetaxel to a prostate cancer patient, 3900 mg PS80 is coadministered on average, prompting recommendations that doses of this excipient be reduced if possible, as with the third FDA-approved taxane, cabazitaxel, which contains 957 mg PS80 per dose, per the Dailymed database when dosed according to 20 mg cabazitaxel/m2 body surface area of an adult (1.84 m2) [7,19].
The article, tellingly, does not mention polysorbate-80 in vaccines at all, and the clinical trial document shows that there were only milligram—not 3.9 grams—quantities of polysorbate-80 in the placebo control for RotaTeq. I could go on and on, but the ingredients in placebos chosen for actual placebo-controlled vaccine trials have well known profiles with respect to what they do and do not do, and all of them are used in amounts so small that they are far below any conceivable toxicity threshold. That’s one huge reason why they are chosen as placebos in the first place! Antivaxxers like to insinuate that, for instance, minuscule doses of sucrose or of sodium phosphate are going to cause adverse events so significant that they can confound a clinical trial when it comes to detecting different frequencies of adverse events in the experimental versus control groups.
On a related note, Aaron Siri and Del Bigtree like also like to invoke their “no saline placebo control RCT” and then claim that the approval of existing vaccines was (and is) all a pyramid scheme in which new vaccines are approved based on comparison with old vaccines, going all the way back to original first-generation vaccines that, according to them, didn’t use the “right” placebo comparator, which to them is always a saline placebo control. Dealing with that one will require considerably more effort because it would require looking up all the licensure studies. Brandolini’s Law, which states that “amount of energy needed to refute bullshit is an order of magnitude bigger than that needed to produce it,” strikes again, or, as Jeff Yates put it about a deceptive video about COVID-19, “The guy filmed himself in his living room, got millions and millions of views, made everybody freak out and it took me two or three days to fact-check.” (From my perspective, Brandolini was an optimist; it often requires more like two or three orders of magnitude more energy to refute bullshit like this.)
“No saline placebo controlled” vaccine studies: Anantivax distortion that never dies
I started out this post by repeating that “everything old is new again” in terms of antivax misinformation during the pandemic. I realize that I repeat this observation practically ad nauseam, but that doesn’t make it even less true, particularly since RFK Jr. seized such a huge platform to resurrect antivaccine misinformation, half-truths, and lies that he’s been repeating for 18 years. It also frustrates me that after all these years the FDA and CDC haven’t fixed simplistic definitions that can be weaponized by antivaxxers, such as their definitions of “placebo,” to make them more consistent with what science actually says. I realize that they likely chose those definitions for the sake of simplicity, to be easily understandable by the lay public, but unfortunately antivaxxers make them even more easily misunderstandable by the public. Such simplistic definitions leave a huge opening for antivaxxers like RFK Jr. and Aaron Siri to selectively cite them instead of more complete and accurate definitions of a placebo or what the FDA, ethics, and science require in a valid control group for an RCT of vaccines. The important thing to know about appropriate placebo comparators for an RCT is that they do not need to be “completely inert.” (Nothing is “completely inert.”) They just need to be “inert” with respect to the outcomes being measured in the RCT. Dr. Offit has referred to placebos for vaccine RCTs as “immunologically inert,” which is as good a definition of a placebo for a vaccine trial as I can think of.
Basically, to delude yourself into thinking that there is clinical equipoise in a vaccine RCT using a saline placebo as control for anything other than a vaccine against a disease that doesn’t already have a licensed safe and effective vaccine against it, you have to believe fervently, as antivaxxers do, that vaccines are more harmful than the diseases against which they protect. That is why, no matter what comparators are used in the control groups in RCTs of vaccines, antivaxxers will find a way to falsely portray them as being a nefarious plot to “hide” adverse events in the vaccine group and demand The One (Impossible) Study To Rule Them All. Taking one example and just with respect to the MMR-II vaccine, such a study would require:
So, starting with the first ingredient on the list, we’d have to have a group that received every single vitamin injected, every single vitamin in combination with every single other vitamin, in combination with multiple of the other vitamins, etc. Let’s say there are four different vitamins (A, B, C, D). We’d have to have an A only group, a B only group, a C only group a D only group, an A/B group, an A/C group, an A/D group, a B/C group, a B/D group, a C/D group, an ABC group, an ABD group, an ACD group, a BDC group, and an ABCD . That’s 15 comparison groups for four ingredients. Then, we’d have to have a group for each vitamin in combination with each other ingredient – vitamin A combined with sucrose, B with sucrose, D with sucrose, etc. Then we would have to test each other ingredient in various combinations with other ingredients. Again, thousands of groups, each having hundreds or thousands of children to cover all combinations mentioned above.
The above breakdown of possible combinations would have to be repeated for every vaccine, for every booster, and for every possible combination of boosters.
Dr. Paul Offit reminds us what the potential cost of demanding placebo controls when they are not appropriate, going back to the largest saline placebo-controlled clinical trial of a vaccine ever undertaken, in which the use of a saline placebo control was considered ethically appropriate by most, but not all, scientists:
The casual cruelty expressed by ICAN’s lawyer can also be found in an event that occurred almost 70 years ago. In 1954, 420,000 first and second graders in the United States were inoculated with Jonas Salk’s inactivated polio vaccine; 200,000 were inoculated with salt water. It was one of the largest placebo-controlled trials of a medical product in history. Jonas Salk didn’t want to do it. He couldn’t conscience giving a saltwater shot to young children when as many as 50,000 were paralyzed by polio and 1,500 died every year. When the trial was over, the vaccine was declared “safe, effective, and potent.” Church bells rang out; synagogues held special prayer meetings; department store patrons stopped to listen to the results of the trial over loudspeakers. How did we know that Jonas Salk’s polio vaccine was effective? We knew because 16 children died from polio in that study—all in the placebo group. We knew because 34 of the 36 children paralyzed by polio in that study were in the placebo group. These are the gentle heroes we leave behind.
I suspect that none of the parents who volunteered for Jonas Salk’s polio vaccine trial were hoping their children were in the placebo group.
Casual cruelty indeed. Because the Salk polio vaccine was the first of its kind, science did require a placebo control (although an argument could have been made for using historical controls as a comparator), but there was a high cost. It is a cost that should never be considered lightly or accepted when not absolutely necessary. Again, ethics must always win out when designing an RCT. Antivaxxers think nothing of demanding massive clinical trials testing all potential combinations of vaccine ingredients against each other—and against a saline placebo, of course!—with no thought to ethics, potential harms to subjects, practicality, feasibility, and cost.
Finally, I will conclude by emphasizing once again that, contrary to the fevered rantings of antivaxxers,, there is nothing nefarious in there existing a number of vaccines that were tested against older versions of a vaccine against the same disease or that were not tested against a “saline placebo.” That’s literally how ethical clinical trials work. They balance scientific rigor against ethics, which often leads to compromises because ethics always trumps rigorous clinical trial design. If that weren’t the case, then we’d be leaving cancer patients untreated so that we can test new chemotherapy drugs against an “appropriate” placebo control. Moreover, an “appropriate” placebo for an injectable drug or vaccine does not have to be saline. There are many more placebos that are entirely appropriate to use as a control in a vaccine RCT other than just saline.