Covid mRNA Injections: Two Surgeon Generals Now Stand on the Right Side of History. Florida and Louisiana are Leading the Way
For years, Florida’s Surgeon General Dr. Joseph Ladapo stood as the only state Surgeon General warning about the dangers of the COVID mRNA injections. Now, Louisiana’s Surgeon General Dr. Ralph Abraham has joined him:
.
.
.
.
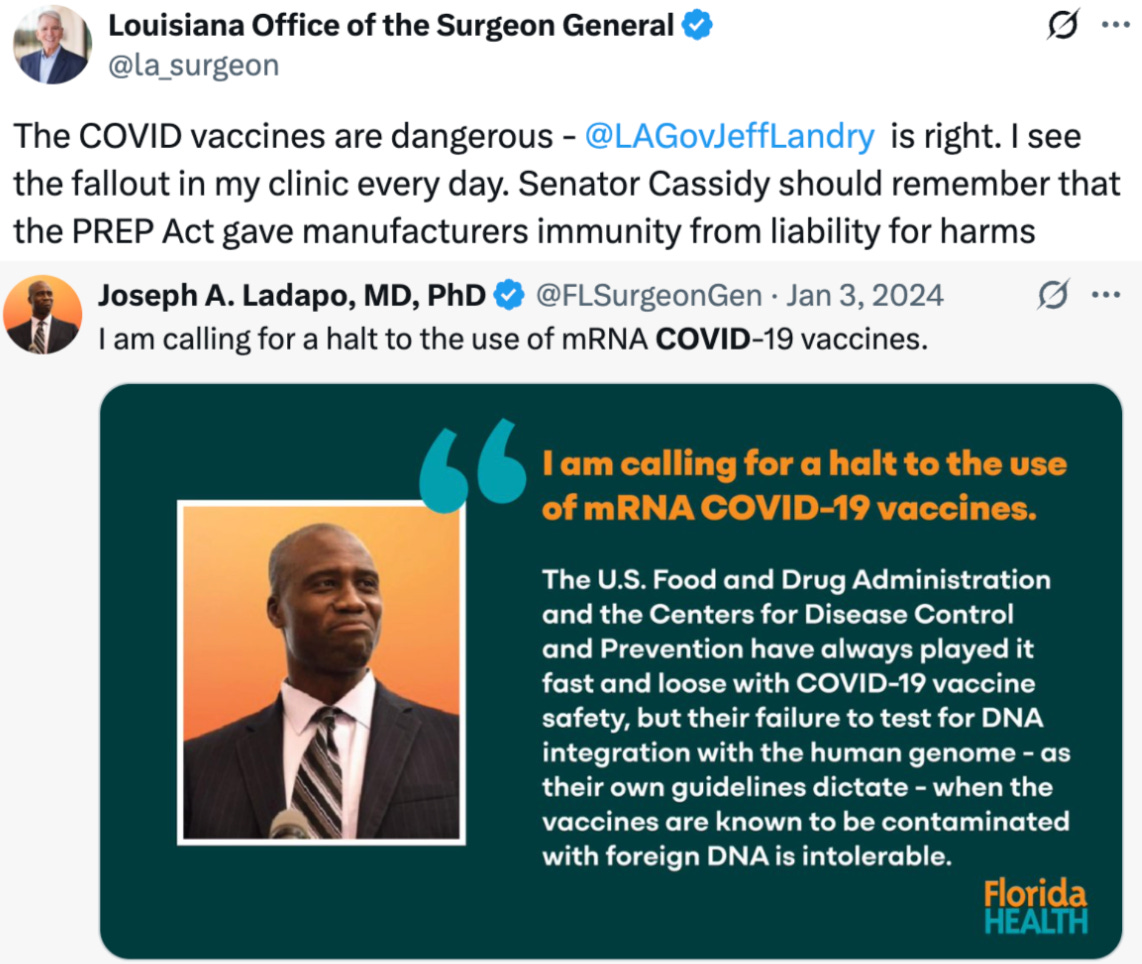
.
Their …
The post Covid mRNA Injections: Two Surgeon Generals Now Stand on the Right Side of History. Florida and Louisiana are Leading the Way appeared first on Global Research.
Read More



 Heated exchanges between some senators and Health and Human Services Secretary Robert F. Kennedy Jr. during a Sept. 4 hearing amplified confusion about the availability of COVID-19 vaccinations for the fall, with Kennedy misleadingly claiming that “anybody” can still get a vaccine. HHS policies have created roadblocks to vaccine access.
Heated exchanges between some senators and Health and Human Services Secretary Robert F. Kennedy Jr. during a Sept. 4 hearing amplified confusion about the availability of COVID-19 vaccinations for the fall, with Kennedy misleadingly claiming that “anybody” can still get a vaccine. HHS policies have created roadblocks to vaccine access.


